Colloids and suspensions
1/45
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
46 Terms
what is a dispersed system
one or more ocmponents dispersed as particles or droplets throughout another component
2 phases vs solutions have 1 phase
what are colloidal dispersions and the size of their particles
dispersions where size of dispersed particles are between 10^-9 and 10^-6 m
suspension particle size >1 um vs colloidal system particle size < 1um
why are suspensions used in formulation of drugs (ie. pros of suspensions over solutions)
taste-masking
not possible to dissolve drug completely within a reasonable volume
drug may be more stable
poorly soluble drugs cannot be made into solutions
prevent degradation - hydrolysis of drug by water
drug may be more stable as a solid so it is dispersed before dispensing into a suspension
properties of good suspensions
particles are small and the same size
homogenous - needs to be evenly distributed throughout the liquid to ensure same dose is given
easy to disperse upon shakinh
what type of properites allows solutions and suspensions to be differentiated
optical properties
what is the Tyndall effect
light scattering by particles in a colloid
light scattering makes the colloidal systems look cloudy/ turbid
true or false: in suspension particles scatter beam of light so path can be seen
true: whereas in solutions, there is very little scattering so path of beam cannot be seen
true of false: the less I (turbidity), the less turbid the sample is so the lower the conc of dispersed particles
false: the less I (turbidity), the more turbid the sample is so the greater the conc of dispersed particles
I = Io e-IL
where Io = light intesitiy entering system
I is light intensity exiting system
L = length of sample
particles in colloids will undergo Brownian motion, what is Brownian motion
random movement of dispersed particles throughout the continuous phase
what is the equation for diffusion in colloids
Fick’s Law:
dm/dt = -DA [dc/dx]
which equation is used to determine velocity of sedimentation
Stoke’s Law
V = 2a2g (sigma - p)/ 9n where:
a = particle radius
sigma = density of solid particle
p = density of liquid
n = viscosity of liquid
g = acceleration due to gravity
what is the equation for sediment ratio
R = height of sedimented layer/ initial height of suspension or
R = volume of sedimented layer/ total suspension volume
when making suspensions, what do you add if drug is insoluble in water. how did this agent work
add wetting agents which break down interfacial tension so solid particles are dispersed easily in liquids
liquid needs to be spread around solid for good suspension
what is the interfacial tension
energy barrier which prevents liquid spreading around solid
examples of wetting agents
surfactants, hydrophilic colloids, solvents
clumping → dispersed is achieved when wetting agent is added to clumping state. what is clumping and what happens when you add wetting agents
clumping is when particles stick together
increasing wetting of hydrophobic drug particle leads to decrease in surface tension
clinging → dispersed is achieved when wetting agent is added to clumping state. what is clinging and what happens when you add wetting agents
clinging is when particles adhere (stick) to the container
wetting agents decrease adsorption of particles to container by applying repellent coating
what is aggregation
collection of particles in groups
aggregation of particles in colloids lead to
flocculation (temporary - particles easy to separate)
deflocculation (permanent - difficult to separate)
what is coagulation
when particles are closely aggregated and difficult to redisperse (defloculated systems)
what are flocculation systems
aggregates that have loose structures and particles have a small distance apart and weakly bound in groups
what is caking and what does this mean in terms of dosing
formation of densely packed, non-dispersible layer of aggregates (particles) at the bottom of container that cannot be dispersed again upon shaking
results in patient underdosing (first few doses = no drug) or overdosing (too much drug if taken from the bottom)
true or false: caking cannot be eliminated by reduction in particles size or by increasing viscosity of continuous phase
true: refer to Stoke’s Law
how to reduce caking (hint: want to increase viscosity)
add viscosity enhancer agents which increases viscosity
sedimentation rate (from Stoke’s Law) is slowed
however, caking will still eventually occur with viscosity enhancers
how does flocculating agents reduce caking
encourage floc formation to minimise caking
what creates an electrical double layer in colloidal systems
the electrostation interactions between drug particles and positively or negatively charged ions
flocculation depends on DVLO theory: what does this predict
predicts the stability of charged particles in dispersion to aggregation
VT = VA + VR
VR (potential energy of repulsion) determines whether partially deflocculated system is stable or not
VA potential energy of attraction
VT = total potential energy of interactions
stage 1 of DVLO theory: all particles have a net surface charge
means particles will stay apart
there are counter ions in suspension which will cancel out the surface charges
stage 2 of DVLO theory: encouraging floc formation by controlling net surface charge
use zeta potential to measure how strong repulsion forces are between 2 particles
true or false: the less counterions, the lower the zeta potential
false: the more counterions, the lower the zeta potential because charge cancels out more quickly
can zeta potential be negative and if so when does this occur
yes, when there are too many counterions
what is the ideal zeta potential
want to be close to zero
stage 3 of DVLO theory: what does the diagram look like
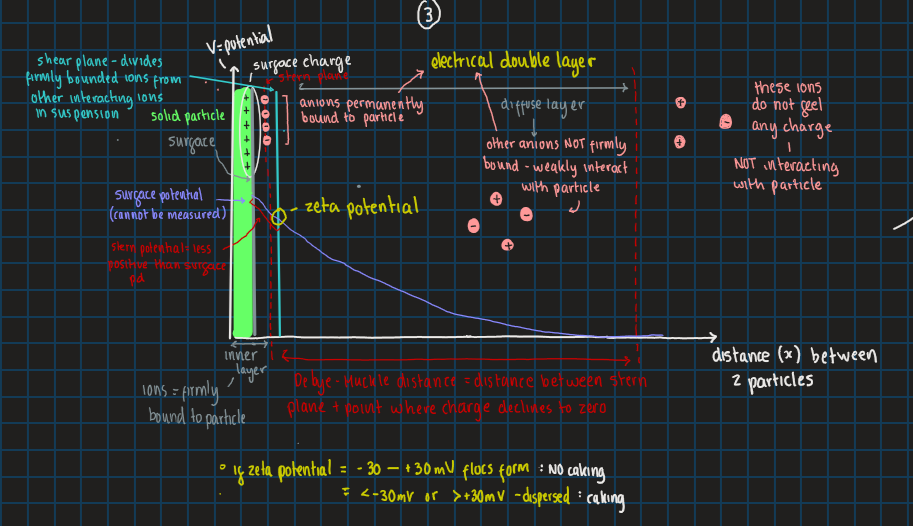
what value of zeta potential will caking occur and not occur
-30 → +30 mV flocs form so no caking
< -30mV or >+30 mV means it is dispersed to caking forms
stage 4 of DVLO theory: what does Vr depend on
surface charge
thickness of double later
Vr affects height of primary maximum and depth of secondary minimum energies
what happens to particles if primary maximum is:
large
small
particles stay dispersed
particles will aggregate
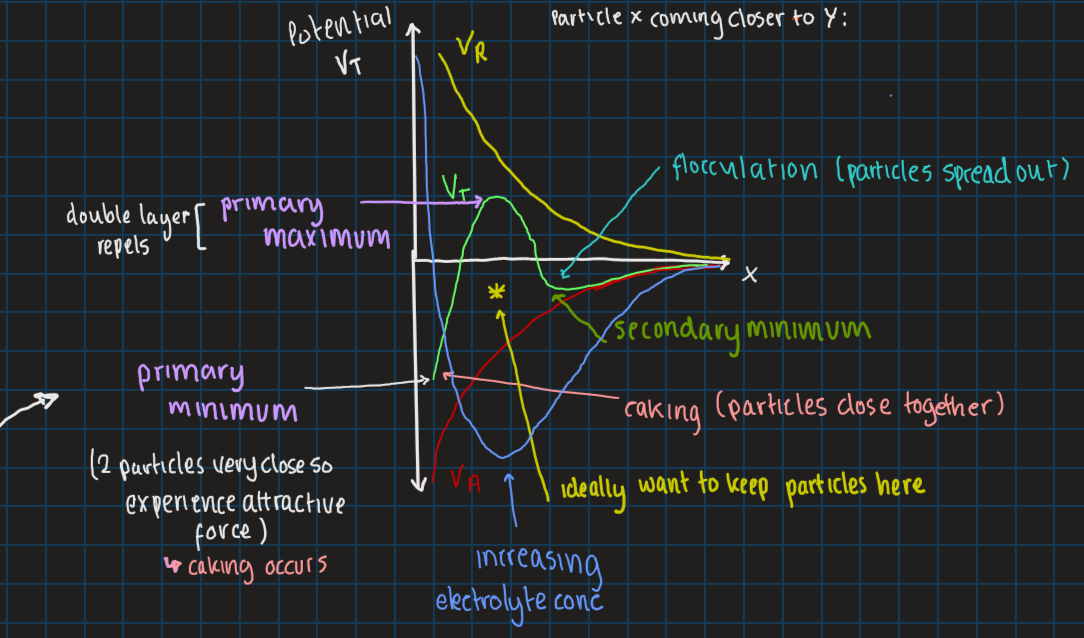
stage 5 of DVLO theory: adding electrolytes (counterions), what will this do
increase Debye-Huckel parameter
decrease thickness of electrical double layer
decrease zeta potential
increase depth of secondary minimum
THIS ALL LEADS TO FLOCCULATION
will increasing electrolyte conc prevent caking and why
yes, because secondary minimum occurs lower on the graph
zeta potential is close to zero → leads to more flocculation
true or false: too much electrolyte can lead to caking
true
changing amount of electrolye will change what
surface charge of drug particle
difference between viscosity enhacing agents and electrolyes
viscosity enhancing agents SLOW down caking process
vs
electrolytes prevent caking
to prevent caking, surfactants are added. what does this do
neutrailises surface charge so repulsion is reduced
adding polymers (chemical groups which interact with particle surface) prevent caking, how does this work
free end of the polymer attaches to another particle and leads to interparticle bridging and flocculation
if there is no particles to interact with, free end of polymer coats the particle which leads to restabilision and deflocculated system
difference between flocculation and deflocculation
in floc vs defloc:
aggregates settle quickly vs sedimentation rate is slowed
liquid is entrapped in sediment so easily redispersed vs slow rate of settling prevents liquid entrapment so compact structure formed (caking), difficult to redisperse
compare properties of flocculated vs deflocculated system
flocculated: particles settle [quickly/slowly] leading to [tightly/loosely] packed sediment with [large/small] volumes of entrapped liquid. this means it is '[difficult/easy] to redisperse
deflocculated: particles settle [quickly/slowly] leading to [tightly/loosely] packed sediment with [large/small] volumes of entrapped liquid. this means it is '[difficult/easy] to redisperse
flocculated: particles settle [quickly/slowly] leading to [tightly/loosely] packed sediment with [large/small] volumes of entrapped liquid. this means it is '[difficult/easy] to redisperse
deflocculated: particles settle [quickly/slowly] leading to [tightly/loosely] packed sediment with [large/small] volumes of entrapped liquid. this means it is '[difficult/easy] to redisperse
why are viscosity enhancing agents useful
they increase viscosity so decrease sedimentation rate
stokes law states that rate of sedmentation is proportion to 1/viscosity