ionic/metallic/covalent bonding
1/13
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
14 Terms
what is ionic bonding
ionic bonding is the bonding between metals and non metals ions and is when electrons are transferred to balance out each other
what are ionic lattices
they are big structures consisting of positive charged ions and negative charged ions which attract each other and make a lattice
properties of giant ionic lattices
they have very strong bonds and high melting point they are solid in room temp and the ions are not free to move. however when they are dissolved in water or in molten form the ions are free to move and they are able to conduct electricity
why do ionic lattices have a high melting point
because when ions have a stronger charge they get stronger electrostatic attractions requiring more energy to break down therefore resulting in higher melting point
what is covalent bonding
covalent bonding is the sharing of electrons between non metal atoms
what is a double bond
a double bond is when one atom is sharing more then one pair of electrons such as o2
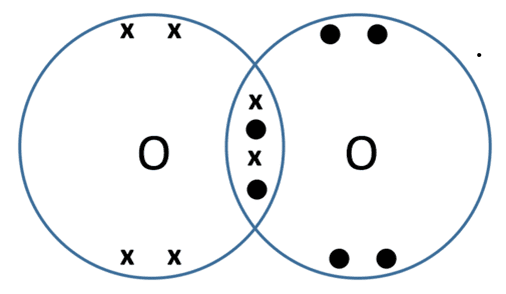
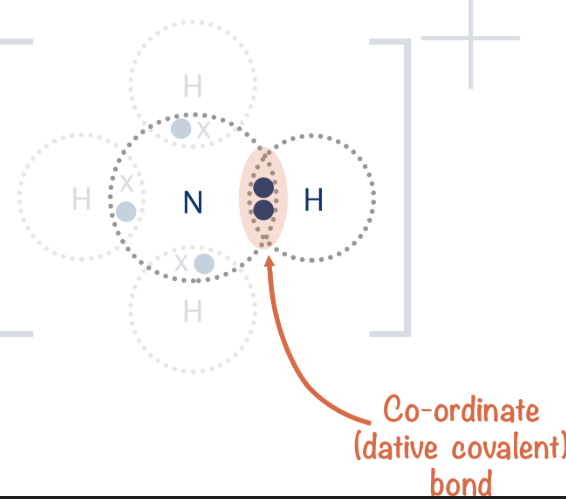
what is a dative bond
a dative bond is when both shared electrons come from the same atom
as you can see in this image both shared electrons come from nitrogen
structure of simple molecular substances
simple molecular substances have strong bonds between atoms but weak intermolecular forces such as graphite so they are hard to break up because of strong bonds but easy to separate because of the weak intermolecular forces
they also have low melting and boiling points and cannot conduct electricity because they don’t have any charged particles
structure of giant covalent substances
giant covalent substances have high melting points and boiling points and have strong covalent bonds between all atoms to form a giant structure of atoms
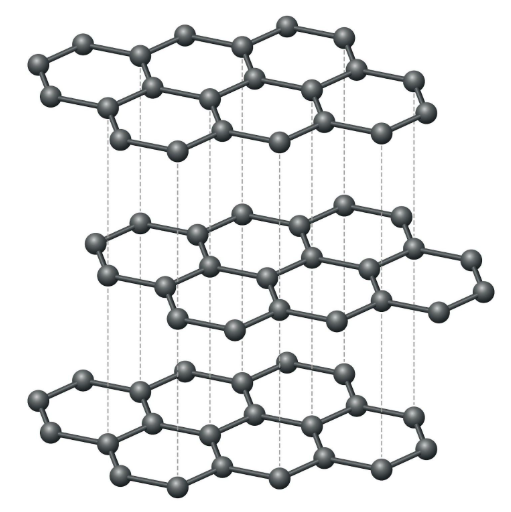
structure of graphite
graphite is a form of carbon and the structure of it is that there are these hexagonal sheets formed by carbon atoms and these have strong bonds but are held together by weak intermolecular forces because of the weak forces they can slide over each other and these electrons in-between are delocalized and can conduct electricity graphite also has high melting and boiling point
structure of diamond
diamond is a very hard material that does not contain any delocalized electrons and therefore cannot conduct electricity and also has a high melting and boiling point as it requires a lot of energy to break down the covalent bonds. the reason it is a very hard substance is because the carbon atoms are arranged in a 3 dimensional tetrahedral shape
what is metallic bonding
metallic bonding is the electrostatic attraction between the nuclei of positive metal ions and delocalized electrons that are free to move
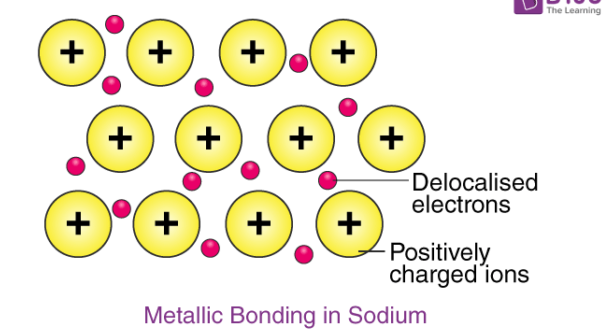
giant metallic structures
giant metallic structure consists of a giant lattice with layers of positive metal ions and delocalized electrons forming strong non directional electrostatic attractions. these layers can also slide over each other making them malleable and ductile
do metals have high or low melting points
they have high melting and boiling points because it requires a lot energy to break down the strong electrostatic attraction between the positive metal ions and delocalized electrons