C3.3 The mole and the Avogadro constant
0.0(0)
0.0(0)
Card Sorting
1/3
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
4 Terms
1
New cards
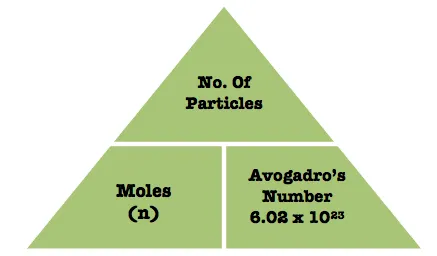
What is a mole?
Unit for measuring the quantity of a chemical substance.
Contains 6.02×1023 particles (Avogadro’s constant)
2
New cards
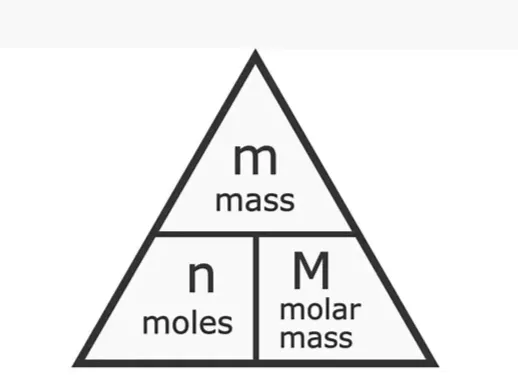
Mole with molar mass
3
New cards
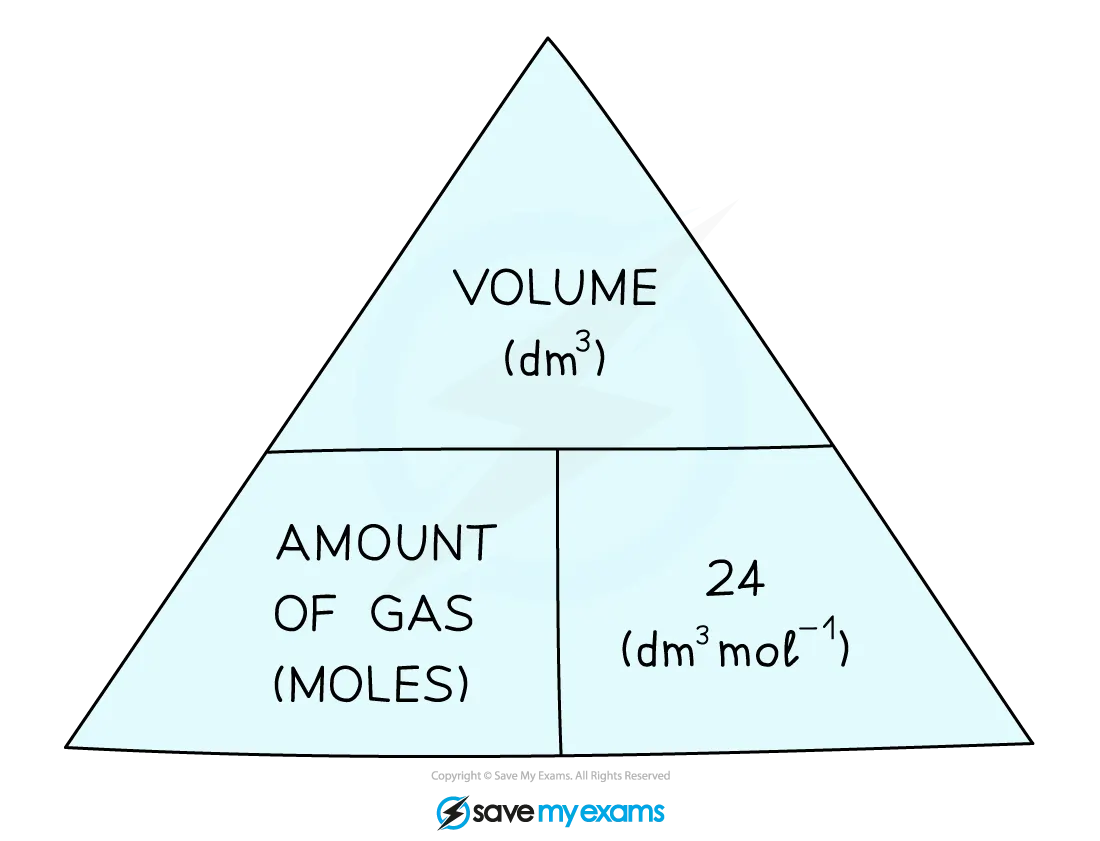
Mole with volume
1 dm3 = 1000 cm3 (dmx1000)
1cm3 = 0.001dm3
4
New cards
How to calculate number of particles from a mole?