The Arrhenius Equation
1/9
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
10 Terms
Rate and temperature
increase temperature = particles have more kinetic energy so they speed up and collide more often
also means more reactant particles will have the required activation energy for the reaction to a greater proportion of collisions will result to a reaction happening
= increased rate
Temperature, rate and rate constant
changing temp ≠ effect on concentration of reactants
so you’d have to change the rate constant
at a higher temp = higher rate constant
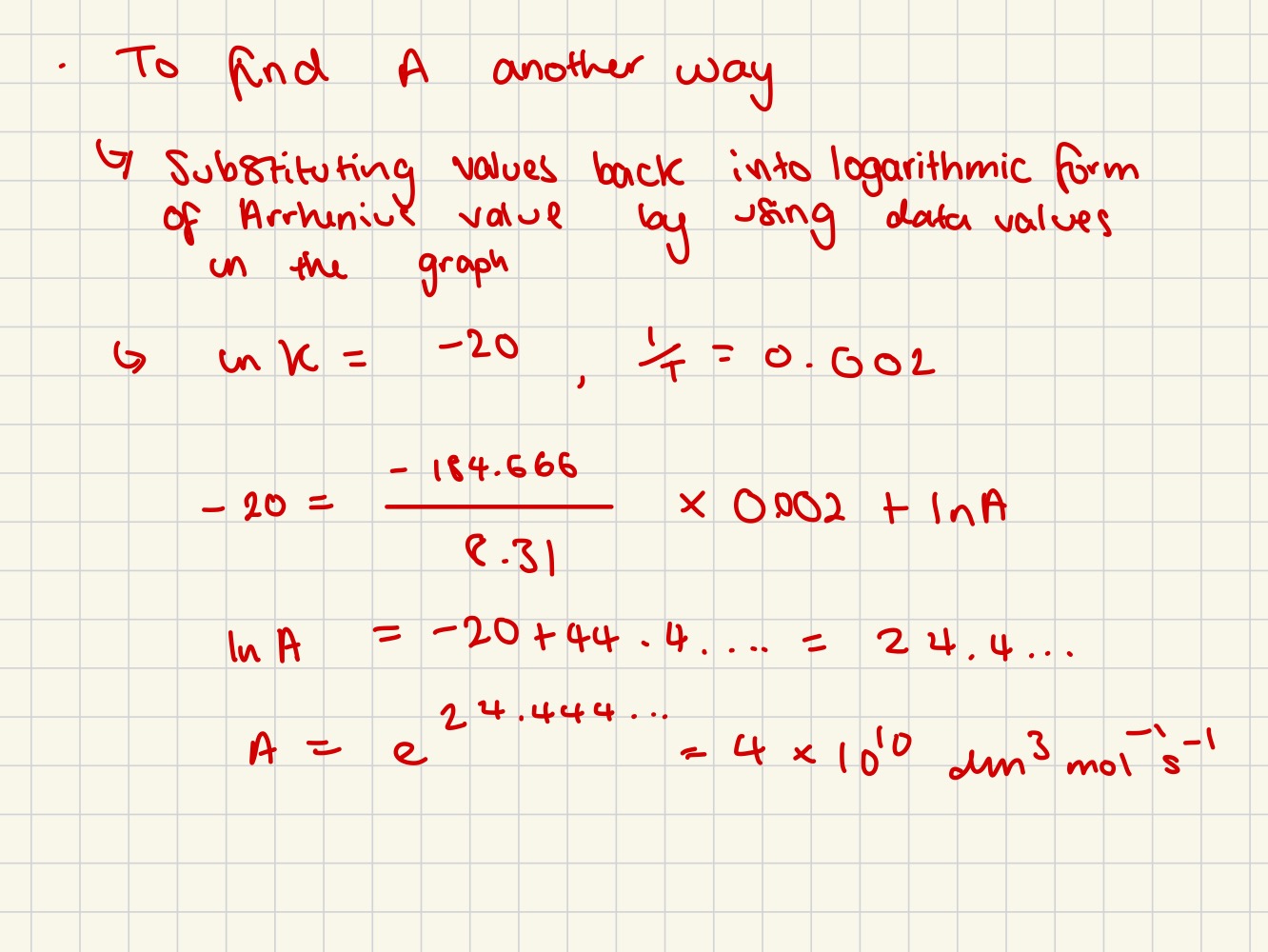
Arrhenius Equation
The Arrhenius equation expresses the relationship between the rate constant (k) of a reaction with activation energy and the temperature (T)
A is the pre-exponential factor, Ea is the activation energy, and R is the gas constant.
Rate constant and activation energy
activation energy gets bigger = rate constant gets smaller
Rate constant and temperature
temperature increases = rate constant increases
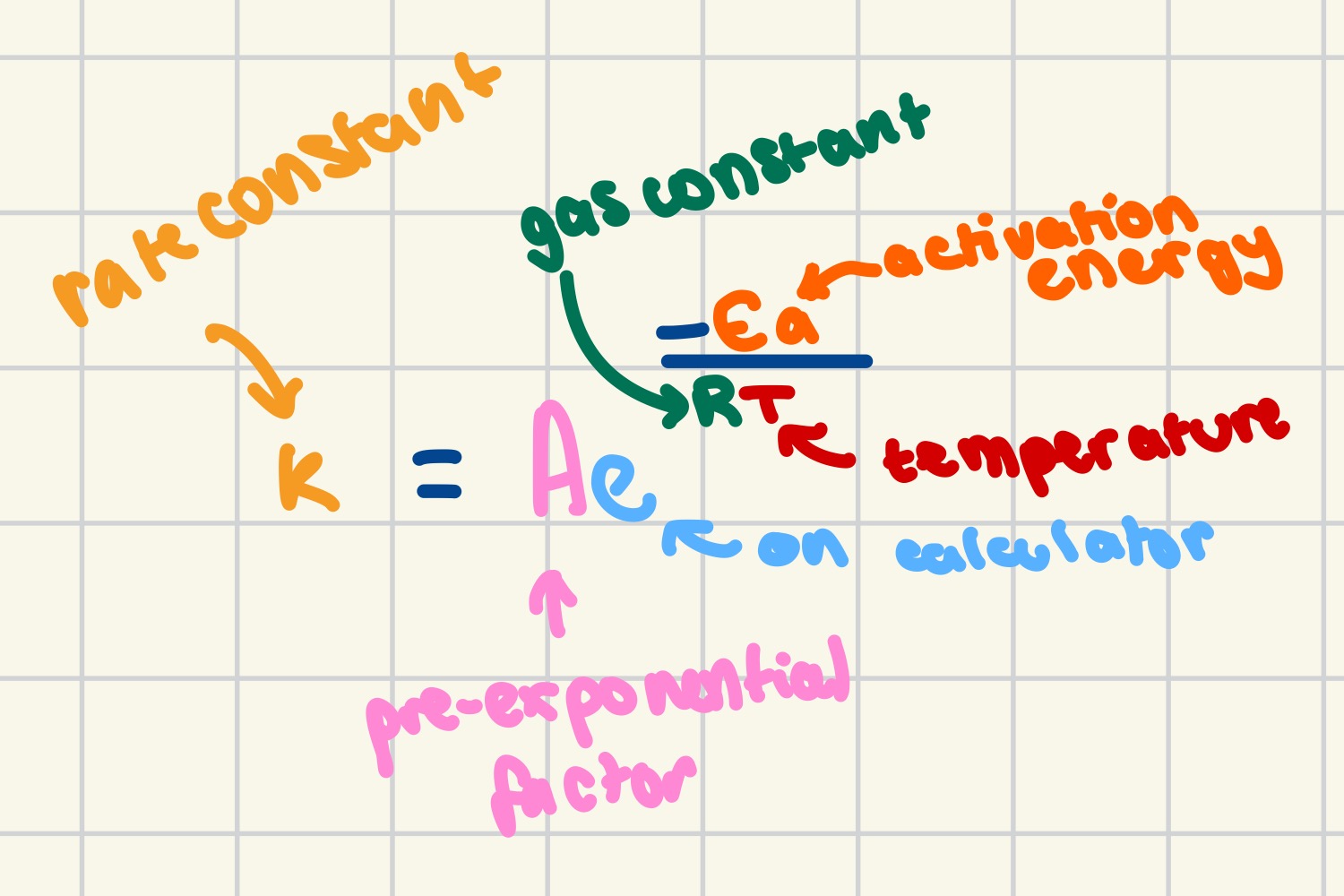
rearranging the Arrhenius equation
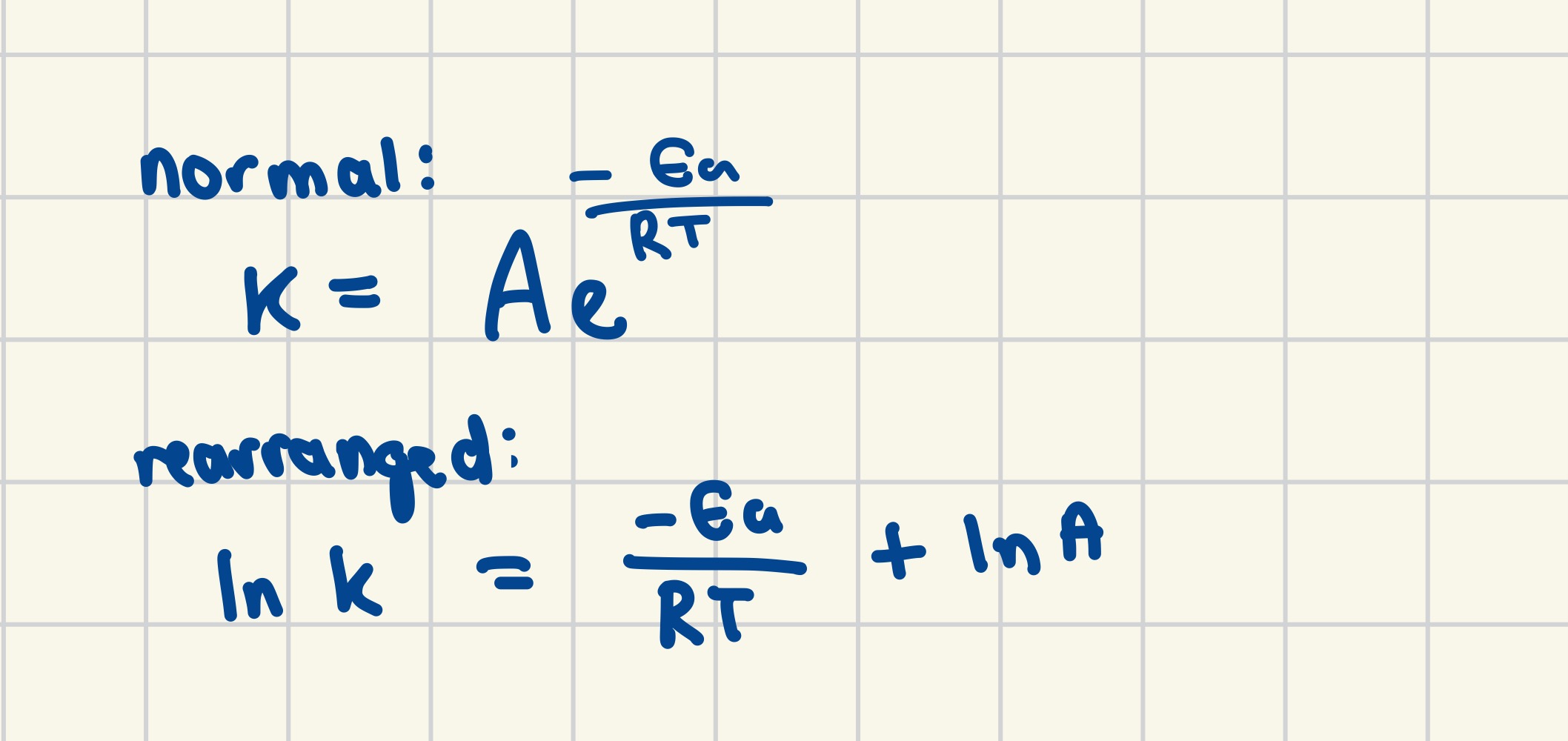
Arrhenius plots
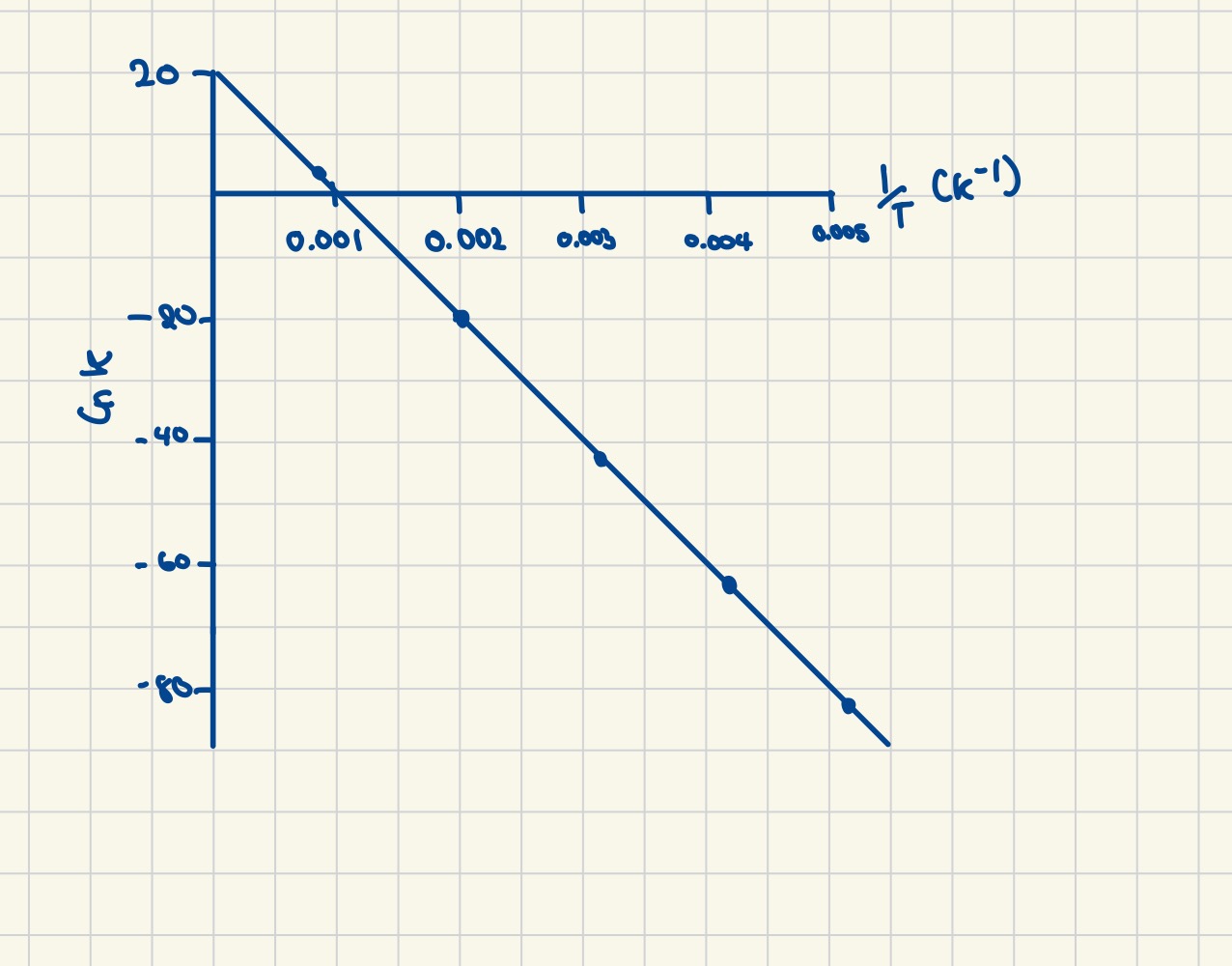
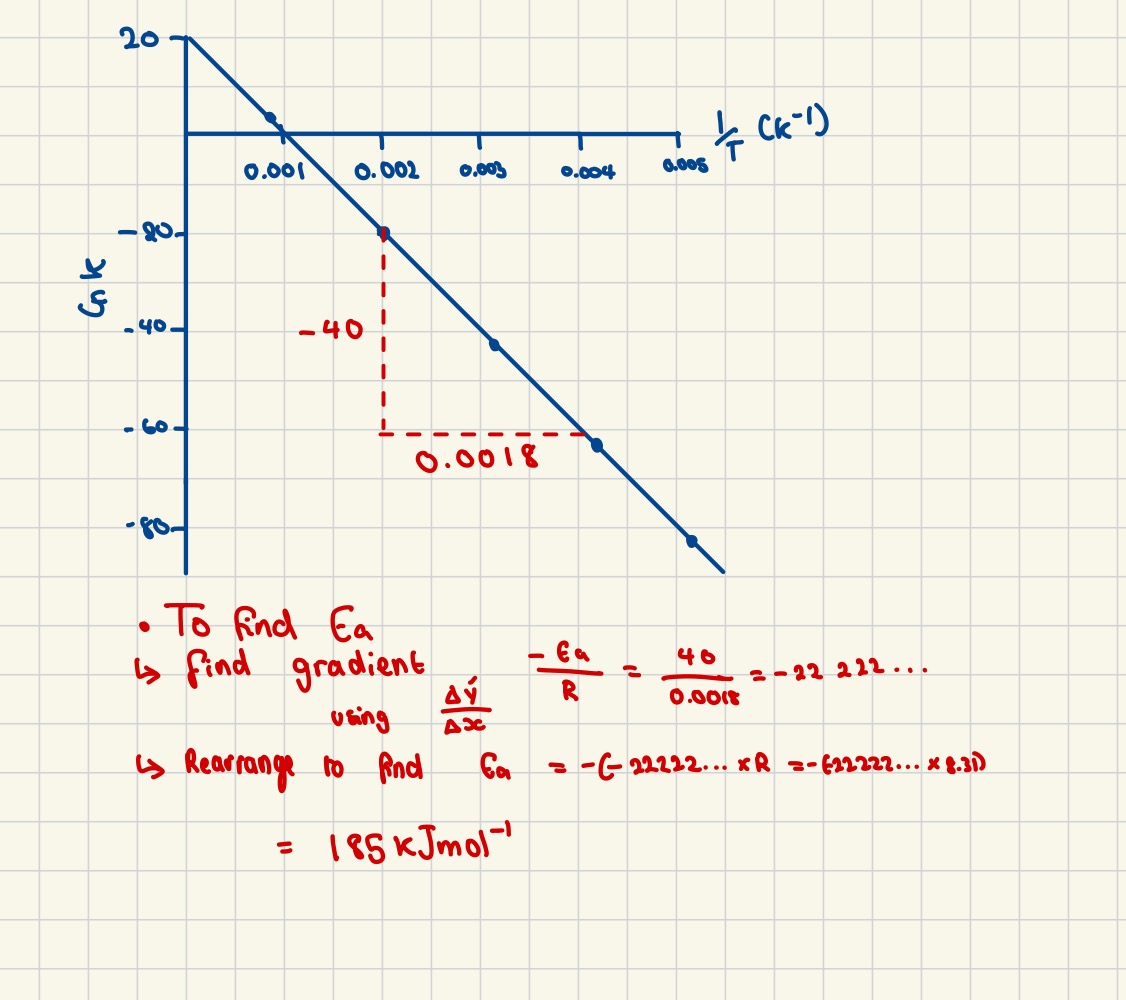
Finding the value of Ea
Finding the value of ln A
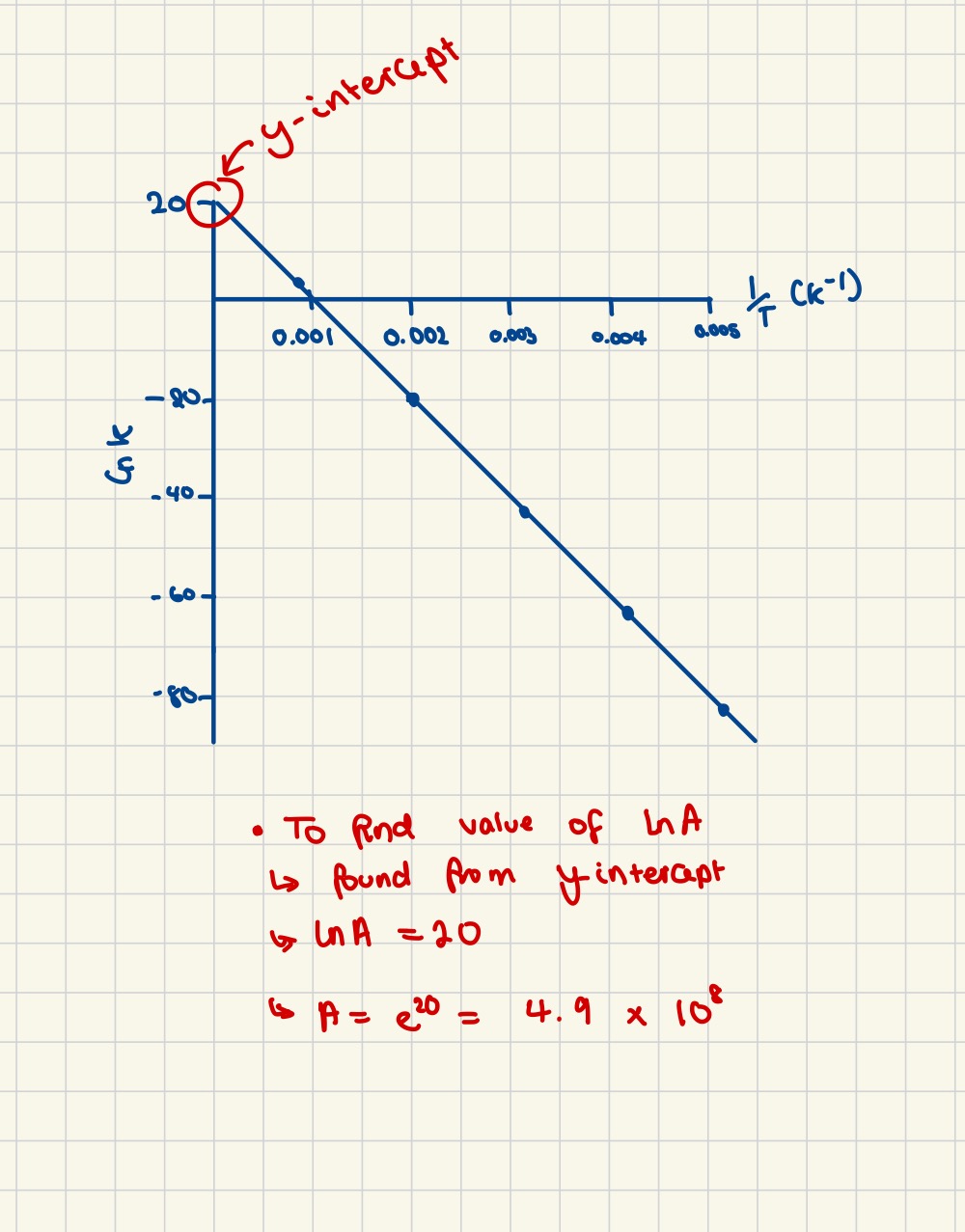
Finding the value of A (another way)