Reaction Mechanisms
0.0(0)
0.0(0)
Card Sorting
1/7
Earn XP
Description and Tags
O chem
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
8 Terms
1
New cards
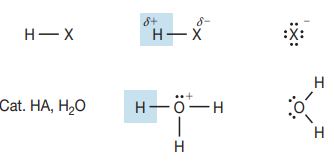
Nu = X or OH
double bond attacks H
Markovnikov addition - not stereospecific
Anti-Markovnikov with H2O2
racemic mix
2
New cards
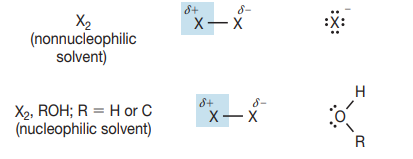
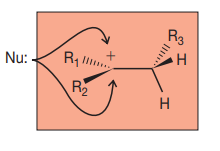
Anti-Mark addition
Form cyclic intermediate ion
trans-aklene = meso comp
cic-alkene = pair of enantiomers
(if reacting with H2O, O is more electronegative thus attaches to more highly substituted C because it has a more positive charge)

3
New cards
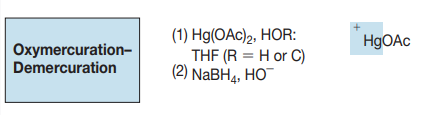
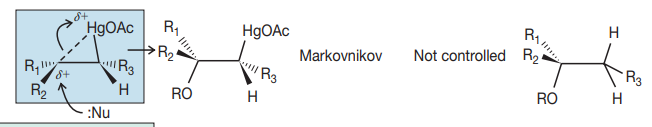
Cyclic intermediate
syn addition
Markovnikov addition
not controlled stereochem.
4
New cards
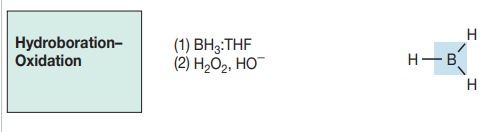
cyclic intermediate
anti- markov addition
syn addition
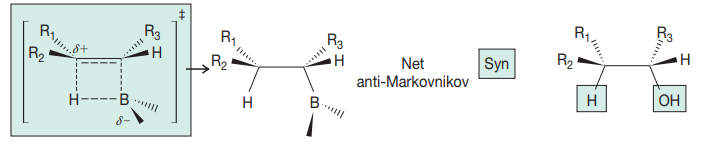
5
New cards

Syn addition of two OH groups
Mix of enantiomers

6
New cards
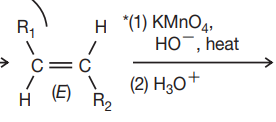
di sub = ketone
mono sub = carboxylic acid
uni sub = CO2
7
New cards
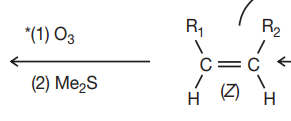
di sub = ketone
mono and uni sub = aldehyde
8
New cards
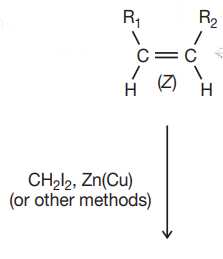
cis alkene = cis alkane
trans alkene = trans alkane