Arrhenius etc
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
12 Terms
what is the relationship between ln k and T?
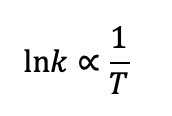
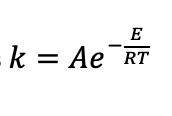
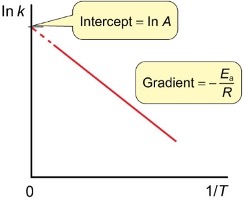
Arrhenius graph
intercept? gradient?
what is the collision theory equation in words?
rate = rate of collisions x fraction of collisions with enough E to react
what is rate of collisions related to?
size of molecules and it is weakly temperature dependent
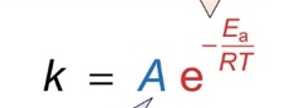
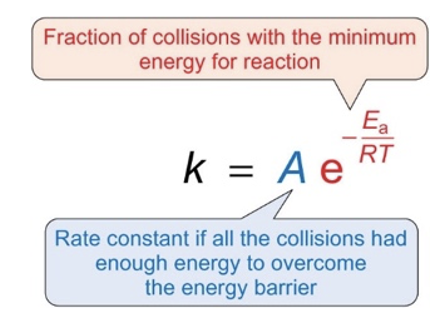
what do the different terms in Arrhenius equation relate to?
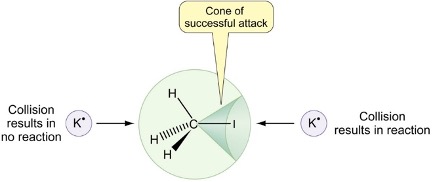
which collisions result in reactions (diagram)?
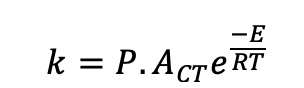
what is P in collision theory equation?
steric factor, accounts for orientation effects
assumed that not all collisions with sufficient energy result in product formation
transition state theory equilibrium
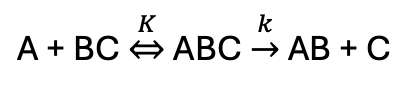
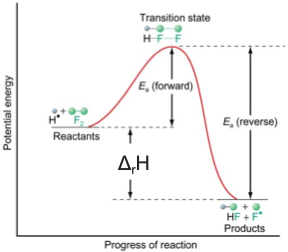
where is transition state on energy profile?
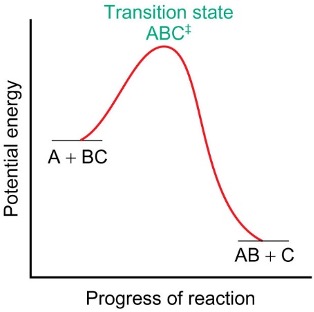
why doesn’t catalysis alter POE?
lowers activation energy for both forward and backward reactions (speeds up rate)
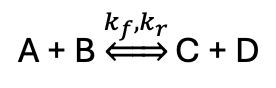
how to calculate K from rate coefficients?
rate of forwards and backwards reactions are equal
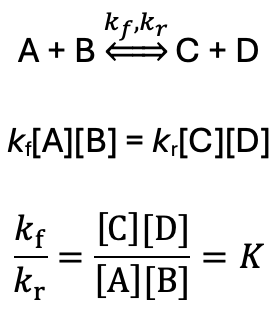
on an energy profile, where is Ea reverse?