ligand substitution reactions
1/10
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
11 Terms
what would happen if one similarly sized ligand is substituted for another? give an example:
no change in coordination number
no change in shape
change in colour
e.g. [Co(H2O)]2+ + 6NH3 → 6H2O + [Co(NH3)6]2+
![<ul><li><p>no change in coordination number</p></li><li><p>no change in shape</p></li><li><p>change in colour</p></li><li><p>e.g. [Co(H<sub>2</sub>O)]<sup>2+</sup> + 6NH<sub>3</sub> → 6H<sub>2</sub>O + [Co(NH<sub>3</sub>)<sub>6</sub>]<sup>2+</sup></p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/cc977762-59e8-4820-a756-81adced8ff23.png)
what would happen if one different sized ligand is substituted for another? give an example:
change in coordination number
change in shape
change in colour
e.g. [Cu(H2O)6]2+ + 4Cl- → 6H2O + [CuCl4]2- - Cl- ligands are bigger than H2O and NH3 ligands so the shape changes from octahedral to tetrahedral upon substitution (i.e. coordination number changes from 6 to 4)
![<ul><li><p>change in coordination number</p></li></ul><ul><li><p>change in shape</p></li><li><p>change in colour</p></li><li><p>e.g. [Cu(H<sub>2</sub>O)<sub>6</sub>]<sup>2+</sup> + 4Cl<sup>-</sup> → 6H<sub>2</sub>O + [CuCl<sub>4</sub>]<sup>2-</sup> - Cl<sup>-</sup> ligands are bigger than H<sub>2</sub>O and NH<sub>3</sub> ligands so the shape changes from octahedral to tetrahedral upon substitution (i.e. coordination number changes from 6 to 4)</p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/022cc431-b897-4694-8744-b928db4df7c3.png)
what is partial substitution? give an example:
where not all ligands can be substituted e.g. [Cu(H2O)6]2+ (aq) + 4NH3 (aq) → [Cu(NH3)4(H2O)2]2+ (aq) + 4H2O (l):
here, not all water ligands can be replaced by NH3 ligands :(
shape changes from octahedral → elongated octahedral, w/ 2 H2O ligands occupying the trans positions
![<p>where not all ligands can be substituted e.g. [Cu(H<sub>2</sub>O)<sub>6</sub>]<sup>2+</sup> <sub>(aq)</sub> + 4NH<sub>3 (aq)</sub> → [Cu(NH<sub>3</sub>)<sub>4</sub>(H<sub>2</sub>O)<sub>2</sub>]<sup>2+ </sup><sub>(aq)</sub> + 4H<sub>2</sub>O <sub>(l)</sub>:</p><ul><li><p>here, not all water ligands can be replaced by NH<sub>3</sub> ligands :(</p></li><li><p>shape changes from octahedral → elongated octahedral, w/ 2 H<sub>2</sub>O ligands occupying the trans positions </p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/e5550ad9-d827-42ef-b60a-d2b3badee6d4.png)
describe the structure of haemoglobin w/ reference to it as a transition metal complex:
one haem group contains Fe2+ at its centre which accepts 6 lone pairs from ligands, w/ 4 being from H atoms which form a circle around the molecule - the haem
a globin (type of protein) and either an oxygen atom or water molecule can bind, forming an octahedral structure
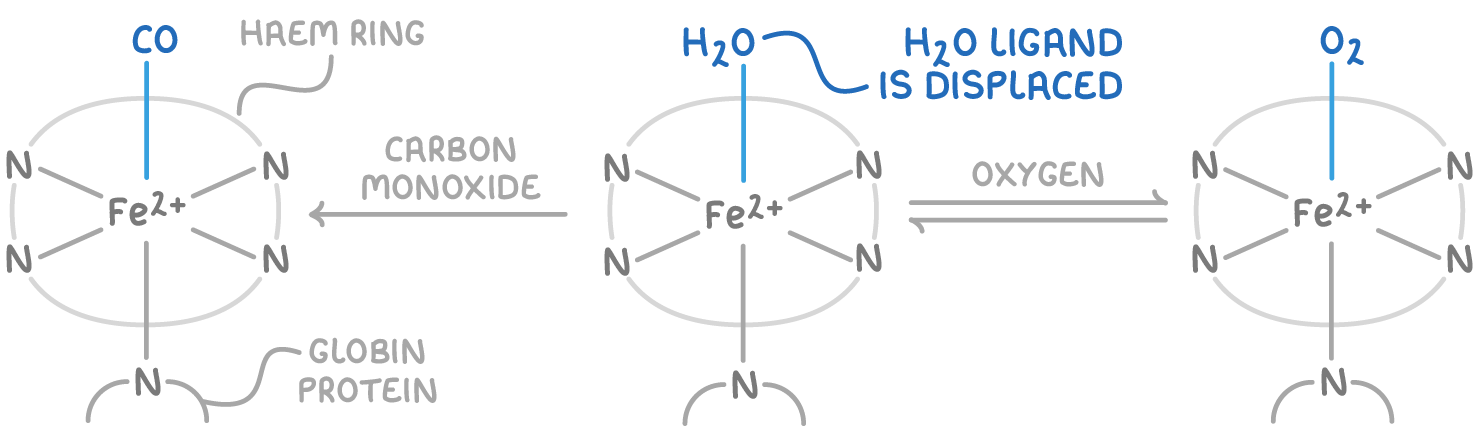
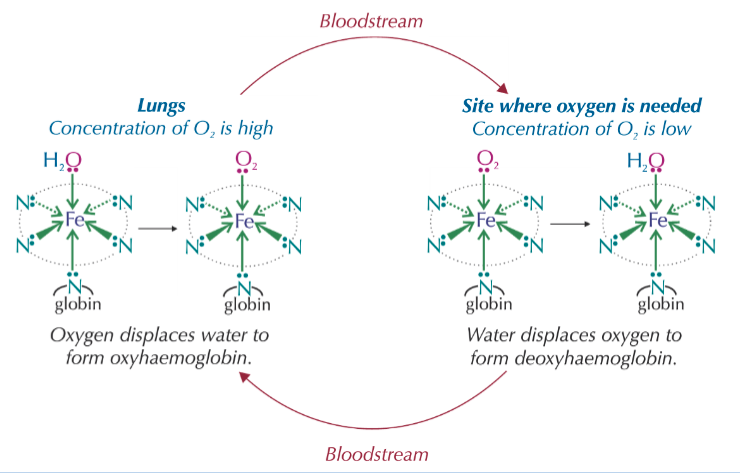
describe the role of haemoglobin in the body with reference to the ligand substitution reactions which occur:
haemoglobin transports oxygen around the body and substitutes it for water where needed
if CO is inhaled, then haemoglobin can substitute the water or oxygen ligands, forming carboxyhaemoglobin
but CO binds more strongly to haemoglobin and doesn’t exchange for water or oxygen ligands
this may therefore starve the body of oxygen, causing headaches/dizziness/death
why is CO toxic?
replaces oxygen co-ordinately bonded to Fe (II) in haemoglobin
are ligand substitution reactions reversible? in what case may this change?
yes - ligand substitution reactions are reversible
unless the new complex is much more stable than the old one
why are EDTA4- and ethylenediamine reactions usually irreversible?
multidentate ligands (such as the above) form much more stable complexes than monodentate ligands
it is difficult to reverse the substitution of a monodentate ligand for a multidentate ligand, as doing so would result in a decrease in entropy
describe and justify the typical enthalpy change for a ligand substitution reaction:
typically low - enthalpy of breaking old coordinate bonds and forming new bonds is fairly similar
explain the chelate effect:
substituting monodentate ligands for multidentate ligands increases the no. of particles in a reaction and ∴ increases entropy - if no. of particles increases, entropy increases
reactions which increase entropy are more likely to occur, which is why multidentate ligands form more stable complexes
it is difficult to reverse the substitution of a monodentate ligand for a multidentate ligand, as doing so would result in a decrease in entropy
give an example of the chelate effect:
reactions replacing monodentate ligands w/ EDTA are very difficult to reverse:
([Cr(NH3)6]3+ + EDTA4- → [Cr(EDTA)]- + 6NH3
2 particles → 7 particles ∴ entropy increases and so stability of complex increases