Exam 4 microbiology Final
1/59
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
60 Terms
What are virulence factors?
They are molecules produced by pathogens (bacteria, viruses or fungi or parasites) that enhance a microbe’s ability to cause disease. By helping them attach, invade and hide, evade and damage tissue.
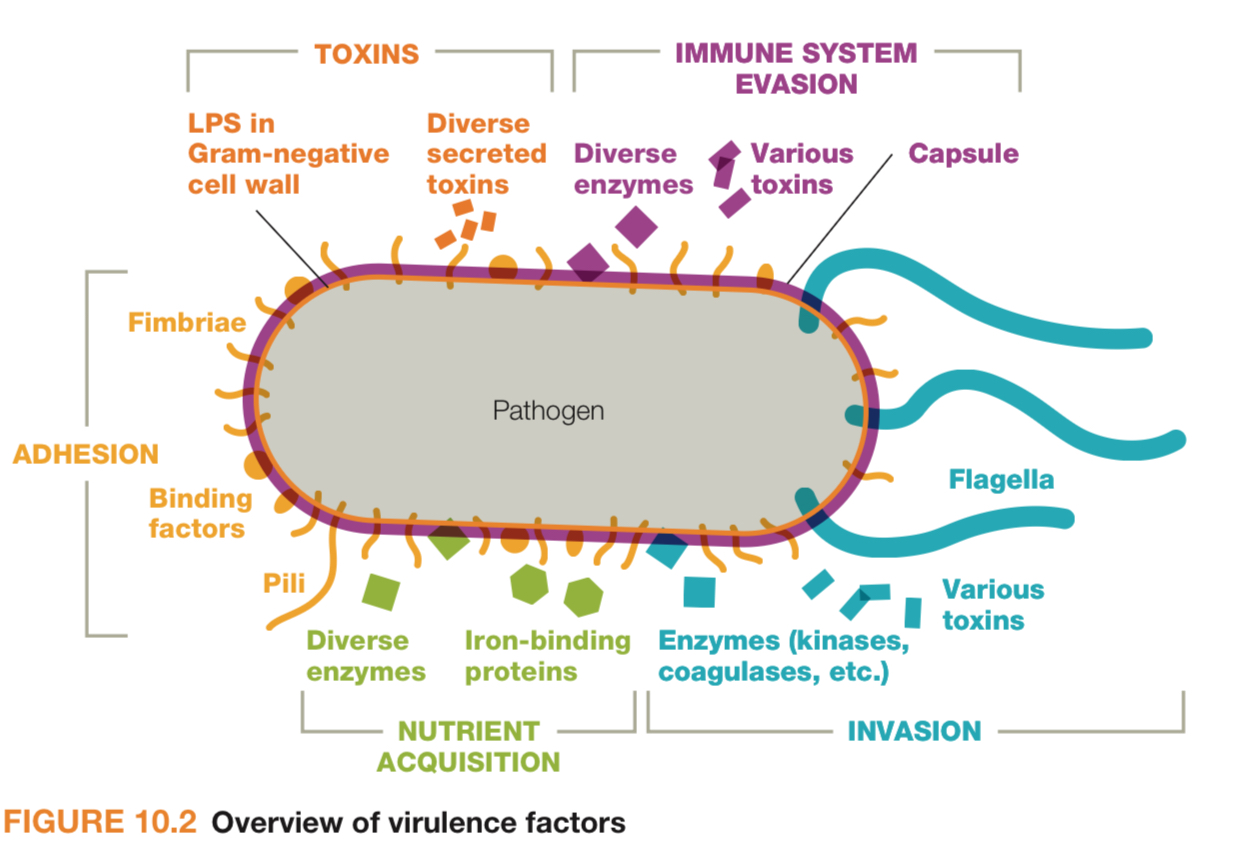
What are some examples of virulence factors?
These factors make a microbe more capable of causing disease (i.e. more virulent)
For Adhesions - Pili, fimbriae in backteria - quantity 1×10^8 cfu
Masking/hiding - Protein A, Capsules, enveloped virus - 2× 10^8 cfu
Evasion - capsules, proteases that breakdown C3 and C5 complement proteins, leukocidins - 3×10^8
Invasion - hemolysins, coagulases, collagenases - 4×10^8
What are acute, chronic and latent infections?
Acute - comes and goes quickly, i.e. flus and colds
Chronic - comes and stays for an extended period of time, i.e. Hepatitis C and HIV
Latent - asymptomatic for a long time until the disease manifests itself in the host, for eg. HIV, Herpes zoster
Persistent - comes and goes - like Genital Herpes
What is a carrier?
Someone who harbors certain pathogens for a long time without experiencing any symptoms. In chronic carriers, the symptoms might emerge time to time (herpes)
What is a zoonotic disease?
When a disease is transmitted from an animal to humans. Eg. rabies, Lyme disease.
How does a disease develop?
Chain of Infection —> Reservoir to Mode of Transmission to susceptible host.
What is a reservoir of infection?
Host for pathogens: Humans (carriers), Animals (zoonoses), Non-living vehicles (contaminated water, food - Vibrio, Salmonella), Soil (Clostridium, fungi (ringworms))
What is a nosocomial infection?
Hospital acquired infection which wasn’t present when they were admitted to the hospital. Examples, are UTI (catheters), pneumonia (ventilators), blood infections (IV’s etc) - all due to poor hygiene, weakened immune system.
Explain Mode of transmission:
Direct contact - touching, kissing, hugging, handshaking etc
Indirect contact - Doorknobs, countertops and bathrooms.
Vehicle transmission - Microbe is on the move through some type of moving inanimate object - waterborne (contaminated water or food), airborne (droplets in dust / air), vector transmission - Mechanical (flies), biological (mosquito bites and infecting)
How are local and systemic infection different?
Local: stays in one area of the body, affects only a specific tissue or organ. Ex. a pimple, a cut infected with bacteria, or strep throat.
Systemic: Spreads throughout the body via the bloodstream or lymph. Affects multiple organs or systems. Example: sepsis, COVID-19 (in severe cases), or measles
List some ways a pathogen can use virulence factors to evade.
Capsules
Proteases that breakdown complement proteins - avoiding opsonization, antibodies
Leukocidins - release endo / exo toxins that damage WBC’s. Leuko (white), cidin (causing it to lyse) by puncturing holes in the membrane.
What are some of the Invasive methods a pathogen uses?
Hemolysins - killing RBC’s
Coagulases - virulence factors produced by bacteria that activate blood clotting around the pathogen to help it hide from immune system.
Collagenases - enzymes produced by some bacteria to break down collagen in bodies —> loosens tissue - deeper invasion - escape local infection and cause systemic infection.
What are endotoxins and exotoxins?
Endotoxins:
made up of Sugar Lipopolysaccharide (LPS)
Produced by Gram (-)
Released upon cell death - part of membrane that breaks
Less potent than Exotoxins
Cause fever, shock, inflammation
NOT heat sensitive
Exotoxins:
Made up of proteins
Produced by Gram (+)
Excreted outside of cell - secreted actively
Very toxic and specific
Targeted (e.g., nerves, gut) - causing fever etc indirectly
Heat sensitive - can have vaccines for it
What is sequential timing? And its relation to concentration dependence?
Sequential timing adds structure and predictability to how the damage unfolds. Certain virulence factors don’t kick in right away they are dependent on the concentration of the pathogen. (Similar to how first innate immunity kicks in and later the adaptive immunity; based on the interleukins and interferons and cytokines etc)
For example: timed sequence is based on pathogens life cycle, environment, or host response
Early stage: Pathogen invades but doesn’t trigger much immune response.
Midway: it produces toxins of enzymes (like collagenases)- Concentration dependent - when pathogens reach a specific density or quorum.
Later: it may breach barriers (like entering the bloodstream), causing sepsis
What are steps of incubation?
Exposure - pathogen enters the body through a portal of entry
Attachment and Colonization - adheres to host cells using pili or surface proteins
Multiplication - replication inside the host
Evasions - Masking/hiding - some release virulence factors like capsules or leukocidins (destroy WBCs)
Invasion - some start the spread to nearby tissues or enter the bloodstream (becoming systemic)
What is an infection ? How is it different than a disease?
Infection is when a microbe enters the body. It may or may not cause any harm. You might or might not show symptoms. You can be infected without getting sick
Disease is when the microbe has started to cause the damage and body starts showing signs of fever, pain , inflammation etc.
What are stages of Disease?
Incubation - upon exposure
Prodromal - when first signs/ symptoms can be observed
Illness - experiencing peak of effects such as coughing, sneezing
Decline - you start to see the decrease of the symptoms perhaps after you start the medication
Recovery - signs and symptoms are completely dissipated
Relate body’s immune response to steps of disease.
Incubation - innate immunity
Prodromal - adaptive immunity
Illness - adaptive immunity + medicine (sometimes)
What is Etiology and how is it different than Pathogenesis?
Etiology is the study of the IDENTIFICATION of the causative agent of a disease (Koch’s postulate). Whereas, Pathogenesis is the study of HOW a microbe causes a disease.
Define type of disease occurrences
Sporadic - occasional cases at irregular intervals. Eg. Ebola
Endemic - continuous occurrence at an expected frequency over a certain periods of time and in a certain geographical location - eg. Colds and flu’s
Epidemic / outbreak - occurrence in a community or region of cases of an illness with a frequency clearly in excess of normal expectancy
Pandemic - epidemic in several countries or continents.
What is Epidemiology?
Epidemiology is the study of how diseases spread, how often they occur, and what factors influence their distribution in populations.
Who is affected
Where and when disease outbreaks happen
Why certain groups are more at risk
How to control or prevent diseases
What is a reservoir?
It’s where a pathogen lives long term and can be transmitted from or maintained as well.
Humans are the reservoir for measles and HIV.
Bats are reservoirs for Ebola and SARS-CoV-2.
Soil is the reservoir for Clostridium tetani (causes tetanus).
What is quorum sensing?
Cell to cell communication between pathogens that allows them to sense how many of them are nearby and once a certain number (quorum) is reached, they switch on group behaviors - like making toxins, forming biofilms, or moving together.
small signaling molecule, as bacteria quantity increases so does the concentration of signal - which, at a certain threshold, binds to receptors and activate genes that lead to group behaviors like producing biofilm or releasing leukocidins (virulence factor)
If a single signal is produced, it has more chances of being destroyed by the immune system so if a larger number is present - it overwhelms the host immune system.
What is a hypersensitivity ? What are its types?
Hypersensitivities are caused by an exaggeration of the host immune response to an antigen. It sees an antigen as an allergen. Type 1 to 3 are driven by antibodies, type 4 is driven by T cells.
Type 1 Immediate - within hours, allergies, involves IgE
Type 2 Cytotoxic - Autoimmune diseases involves IgG/IgM
Immune complex - Inflammation, Involves IgG/IgM, eg Serum sickness, autoimmune
Delayed - T cell mediated. Eg. TB skin test reaction for example.
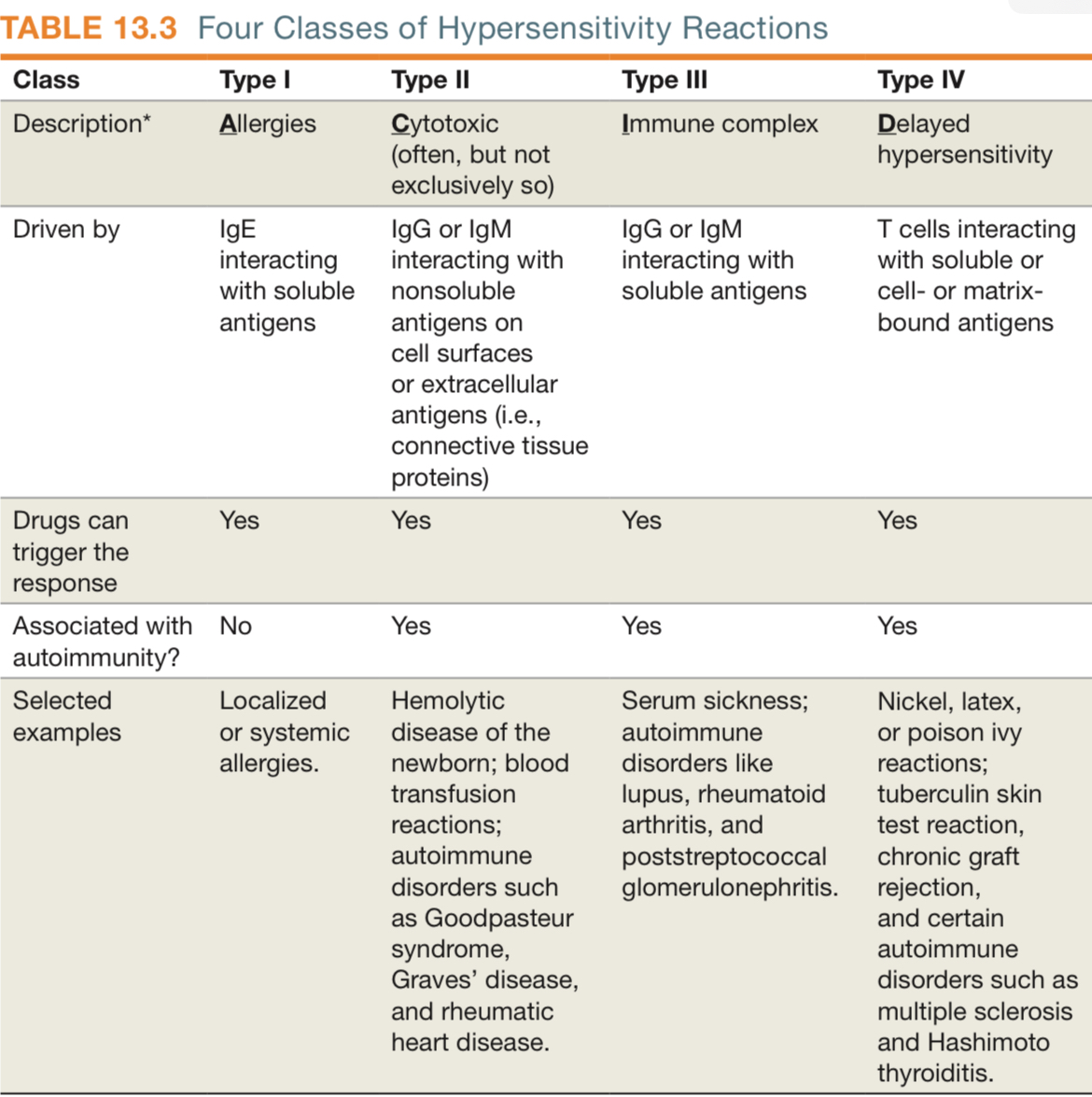
What is Sensitization?
First exposure phase to an allergen (or antigen) that doesn’t yet cause symptoms - but it primes the immune system to respond more strongly the next time. Upon first exposure, the B cells produce the antibodies to specific allergen and upon next exposure, the antigens bind to the antibodies - causing the mast cells to release pro inflammation factors.
Explain Type 1 Hypersensitivity (allergies)
Exposure to pollen or peanuts, no reaction happens, but
B cells recognize the allergen and become Plasma cells to start producing IgE antibodies specific to that allergen. (Priming)
These antibodies bind to Mast cells and Basophils, “arming” them with antibodies on their surface
The person is now sensitized
Next exposure, the allergen binds to these IgE coated mast cells — triggering histamine release - causing allergic reaction.
Explain Type II Hypersensitivity
Causes to have Cytotoxic Reaction due to allergies to some drugs, or mismatched blood transfusions, autoimmune targeting (by mistake or molecular mimicry, genetic predisposition)
First exposure, may be drug binding to cells, immune system recognizes your own cells as foreign and produced IgG or IgM antibodies against these cell bound antigens.
On subsequent Exposure - antibodies bind directly to the antigen on your own cells - activating complement system (mac punching holes in cells), Opsonization (marking cell for phagocytosis) or ADCC (antibody-dependent-cell mediated cytotoxicity) making NK cells destroy the tagged cells.
Examples - Hemolytic anemia, Graves’ disease
Antigens are on your own cells (cell bound)
Explain Type III Hypersenstivity
Produces Immune Complex Reaction - released antigens are soluble and free-floating in the bloodstream or tissues.
What triggers it? - Persistent or chronic infections, autoimmune diseases, inhaled mold spores or antigens from animal danger.
Foreign proteins act as antigens and trigged complex formation and causing Immune system to make IgG antibodies. Upon subsequent exposure - Large antigen-antibody complexes form and deposited in tissues, activate complement and adaptive immunity
Explain type IV hypersenstivity
Involves T delayed cells. APCs activate T cells upon first exposure. T cells multiply and stay in the body (person is now sensitized). Upon next exposure, delayed immune response is triggered (1-3 days) like skin rash or inflammation.
What are key differences in antigen recognition in Type I - Type IV hypersensitivities ?
Type 1: Free-floating antigen triggers IgE production —> binds mast cells —>rapid allergic reaction
Type II: Antigen is stuck on your own cells —> IgM/IgM antibodies binds to the cell —> destruction via complement or NK cells.
Type III: Antigen and antibody form complexe —> deposit in tissues —> activate inflammation.
Type IV: Antigen is processed and presented by APCs to T cells —> delayed T-cell-mediated response (not antibody-based)
What is immunosupression?
When the host immune system is suppressed by an immunosuppressant such as a drug like cyclosporine B which is an anti IL-2 drug - stops interleukin from binding to the receptor.
blocking T cell activation, cytokine release, and T/B cell cooperation, to reduce the immune response.
What are target cells for histamine?
Target cells are the one that get induced by histamine:
Mucosa cells - causes the synthesis of mucous
Lacrimal glands - causes tearing
Nerve cells - causes itching
Blood vessels - causes vasodilation
Bronchial cells - cause bronchoconstriction
What are autoimmune disorders causes by Type 1 -4 Hypersentivities?
Type 1 - Anaphylactic response - allergies. Not any common autoimmune disorders.
Symptoms show up immediately
Mediated by IgE
Anaphylaxis (working against you) vs. prophylaxis (working for you) : Hay fever, asthma, hives, food allergies, and eczema
2 types: Local anaphylaxis (one spot) and Systemic anaphylaxis (all over) aka shock
Type II - Cytotoxic Hypersensitivity:
Autoimmune hemolytic anemia- attacks red blood cells
Hemolytic disease of the newborn (HDN) - Rh+ factor
Goodpasture syndrome - antibodies attack basement membranes in kidneys and lungs
Graves’ disease - antibodies stimulate TSH receptors
Myasthenia gravis - antibodies block acetylcholine receptors at neuromuscular junction
Involved in blood transfusions and some childbirth problems.
Type III - Immune Complex-Mediated Hypersenstivity
Systemic lupus erythematosus - immune complexes affect joints, skin, and kidneys
Rheumatoid arthritis - immune complexes form in joints
Post-streptococcal glomerulonephritis
Serum sickness. Body sees drug as foreign and develop antibodies
Nephritis - inflammatory reaction to the kidneys
Type IV (delayed, T cells mediated)
Type 1 diabetes mellitus - cytotoxic T cells destroy pancreatic beta cells
Multiple sclerosis - T cells attack myelin in the CNS
Graft rejection
Hashimoto’s thyroiditis - T cells destroy thyroid tissue
Contact dermatitis: Poison Oak/Ivy
PPD test for TB
What are different types of grafts in transplant?
Autografts - grafted from one part of the body to another in a patient’s body - usually skin for example.
Isografts - transplanting tissue from an identical twin - genetically identical to host
Allografts - similar genetically to the host but not identical. The close the MHC match, higher chances of success.
Xenografts - interspecies transplant. Pig hear valves into humans etc.
What is autoimmunity?
When the immune system mistakenly attacks the body’s own healthy cells and tissues, thinking they are foreign invaders.
This leads to chronic inflammation and can cause autoimmune diseases like type 1 diabetes, lupus, rheumatoid arthritis, or Graves’ disease.
What is the role of HLA’s ?
Human Leukocyte Antigens are proteins, found on the surface of most cells in the body. They present antigens (pieces of pathogens or abnormal cells) to T cells so the immune system knows what to attack or tolerate.
Organ transplantation - mismatch of HLA’s can cause rejection
How are HLA and MHC related?
HLA is the human version of the MHC (major histocompatibility complex)
MHC is the general term used across all species and in Humans, we call MHC proteins HLA.
What is pathogenicity?
It is the ability of a microorganism to cause disease. It’s different from virulence, which refers to how severe the disease is once the organism causes it.
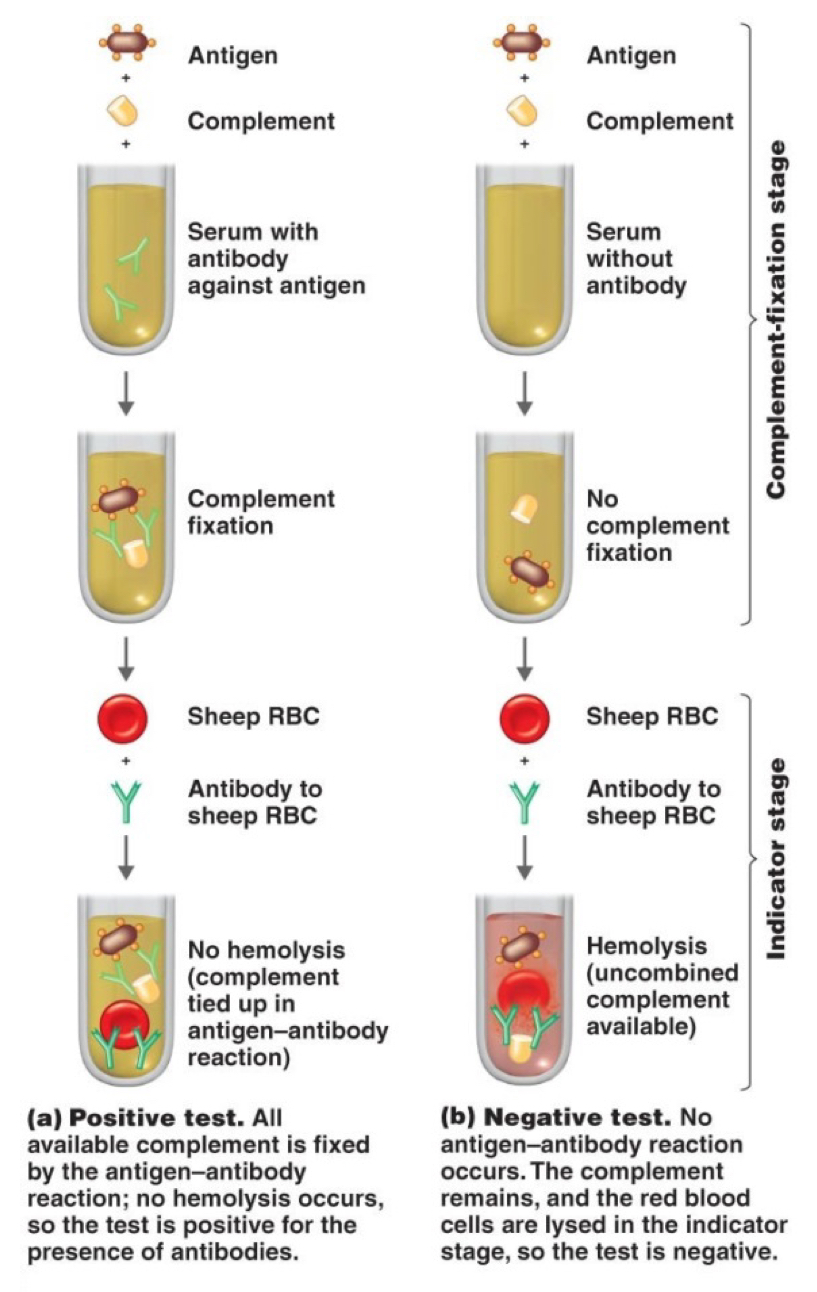
How does Complement Fixation work?
AGs + Complement are added to a serum with or without ABs against AG
If ABs are present → Complement fixation
If ABs are absent → No complement fixation
Afterwards, a signifier, usually sheep RBCs, will be added to check for hemolysis (-) or no hemolysis (+)
How are monoclonal antibodies made?
Antigen is injected into a mouse whose B-cells will produce antibodies for that antigen, these AB producing B cells are then harvested from the mouse’s spleen and combined with myeloma tumor cells to produce a hybridoma cell.
The hybridomas are placed into selective media and are screened for antibody production to be used for ELISAs
Type II Autoimmune Disorders
Same as what causes a type II hypersenstivity except autoimmune disorders is CHRONIC while type II hypersenstivity is acute. Eg. Graves’ disease
Type III Autoimmune Disorder
Caused by the same mechanism as type III hypersenstivity. Ex. Systemic Lupus, heart attacks, and Rheumatoid arthritis
What is type IV Autoimmune Disorder
Chronic condition of type IV hypersenstivity. Examples - Type 1 diabetes, transplantation reactions.
What are Immunodeficiencies?
Immunodeficiency is a Condition in which the immune system’s ability to fight infections and diseases is weakened or completely absent.
There are two main types:
Primary (congenital) - caused by genetic or developmental problems present from birth e.g., SCID - Severe Combined Immunodeficiency (baby has no immune response)
Secondary (acquired) - caused by external factors like infection (e.g., HIV), malnutrition, chemotherapy, or certain medications that suppress the immune system.
What is AIDS?
Acquired Immunodeficiency Syndrome Caused by HIV virus.
Category A - HIV; patient is asymptomatic
Category B - The patient is starting to exhibit the signs and symptoms of AIDS
Category C - The patient is in full blown AIDS. Tc, and Th Cells (macrophages) are targeted.
What is Immunity
Our body’s mechanism for fighting microbes through antibodies and other effector mechanisms like signaling and activation of WBC’s (leukocytes) - macrophages, NK , Tc cells etc.
Differentiate between Active and Passive Immunity
AI - when our bodies synthesize antibodies in response to antigens that we have been exposed to. Keeps memory of the antigens for subsequent exposures. Long lasting protection because of memory and has lag time.
Passive Immunity - When the antibodies are not produced by the body but injected via serum from immune animal or they passed down from a mother to her offspring. Doesn’t have memory so short duration but rapid protection.
What are types of antigens that can lead to antibody production?
A natural or “wild type” (naturally occurring in the environment)
A modified or artificial antigen - one that has been modified and synthesized in the lab
What are the 4 different ways to acquire immunity?
Naturally Acquired Active Immunity - our bodies synthesize antibodies. Eg. Flu / cold (memory + )
Naturally Acquired Passive Immunity - Receiving an antibody from someone who has been exposed to the natural form of the antigen. Eg. Mom to baby (IgG) and IgA (milk)
Artificial Acquired Active Immunity - body develops an antibodies and specialized lymphocytes after being exposed to an artificial form of the antigen. Eg. Flu shot
Artificially Acquired Passive Immunity - Preformed antibodies in immune serum are introduced by injection. Eg. Antivenom.
What are two types of vaccines ?
Live, attenuated; gets strong immune response —> longer lasting and more effective immunity. However, small chance, weakened microbe could evolve and regain some pathogenic traits. Must be refrigerated and it’s hard to produce. (Sabin)
Killed, Inactivated; microorganism is killed by heat, chemicals or radiation. Weaker immune response - requires multiple doses or booster shots. But incapable of causing disease and is easy to produce + store. (Salk)
What are some ways to prevent spread of disease?
Handwashing and public hygiene
If a viral strain is genetically stable - getting vaccinated.
On going vaccinations for Flu etc
Quarantine when you know you have been infected and wear a mask.
What are immunoassays?
Lab tests that use the specific binding of antibodies + antigens to detect infectious disease markers (HIV, hepatitis), hormone levels (TSH, hCG), cardiac markers (troponin), determine blood types, etc.
Common types are ELISA, FIA, RIA, CLIA
What is a Precipitation Test?
Involves soluble (free-floating) antigens. Reach with IgG or IgM antibodies. You mix soluble antigens in the solution with antibodies, if they mix they become insoluble and the antigen-antibody complex precipitates to the top.
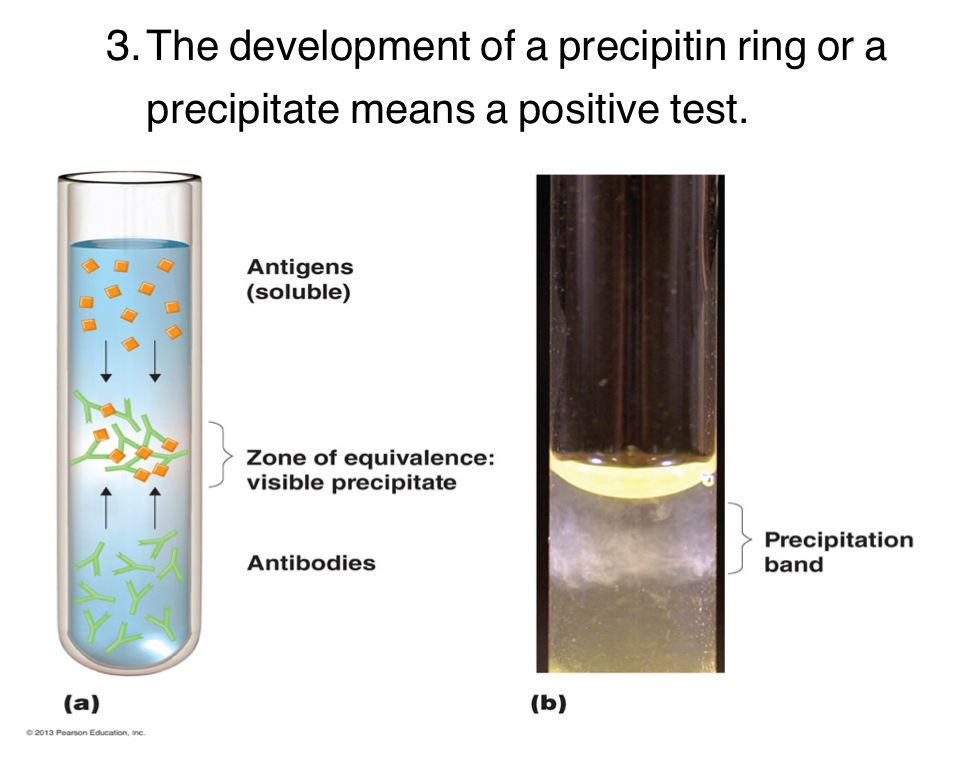
What is Agglutination Test?
Used for blood typing - antigen attached to a solid such as a RBC, observe for aggregates or clumping —> positive test.
Describe F.A.T
Fluorescent-Antibody Test
Identifies microbes in a clinical specimen. Uses antibodies that have been labelled by a fluorescent dye. Can be direct on Indirect and need to use a fl. Microscope to see the fl dye.
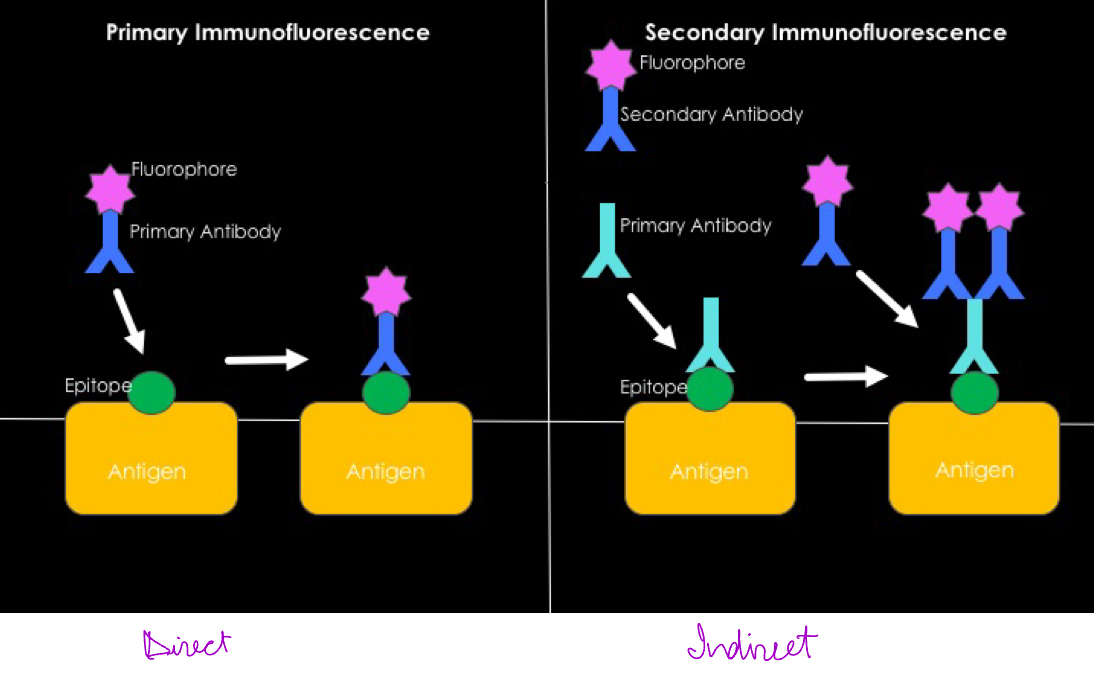
Describe complement fixation test
Occurs when a complement serum protein binds to an antibody/antigen complex. Used to detect small amts of antibody.
Antigen + complement together - if the sample contains antibodies they will bind together to form a complex. And the complement proteins get activated and get used up. Then a red blood cell is deposited, if the cell doesn’t lyse it means positive test for antibodies’ presence.
Describe Neutralization test
Test for antibodies’s effectiveness made in lab
Blocks harmful effects of a bacterial toxin or virus. If antibodies are present, it tests if they can neutralize the toxin therefore preventing agglutination.
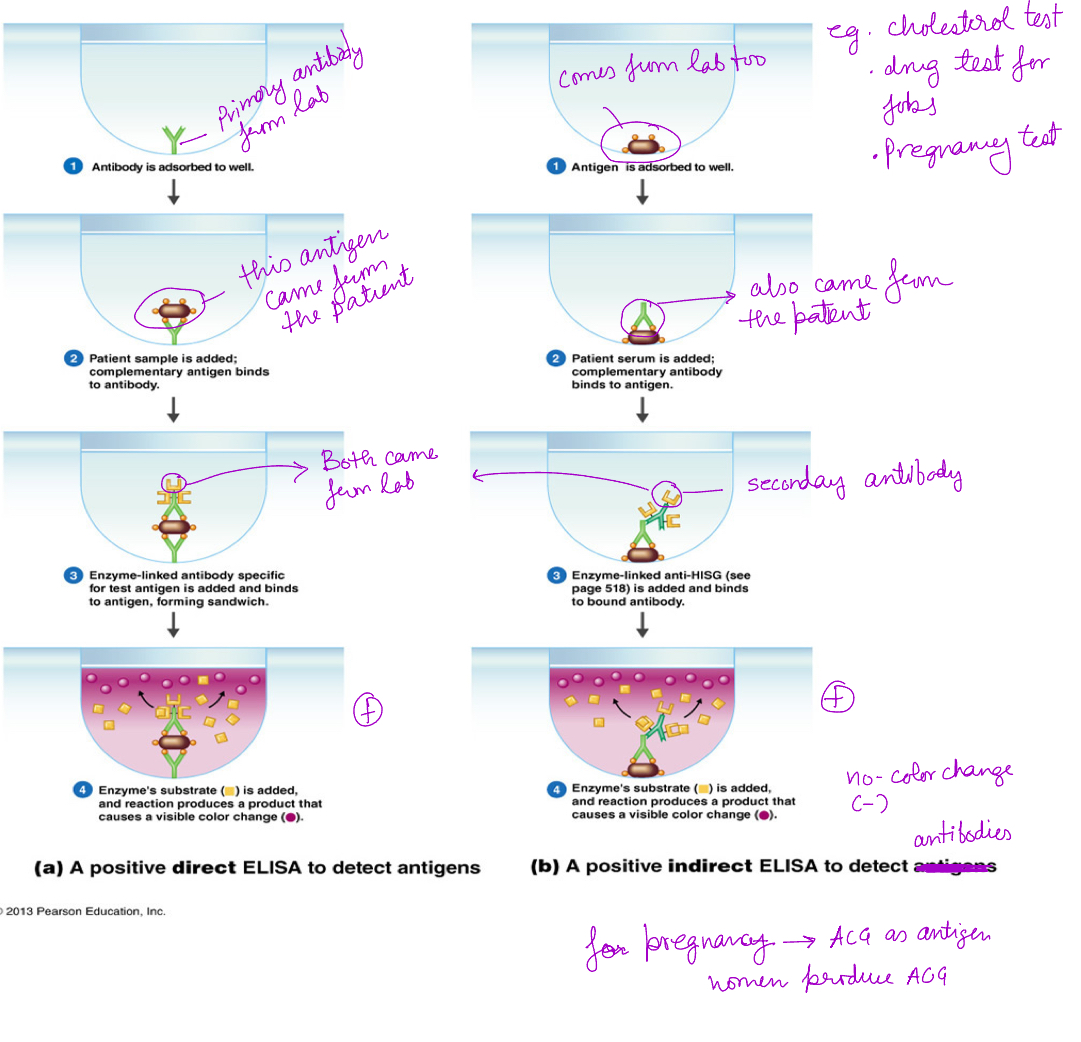
What is ELISA?
Enzyme Linked Immunosorbent Assay
Direct ELISA detects antigens: AG is bound to the plate, and a labeled primary antibody binds directly to the antigen. Faster and less sensitive, used for detecting high concentration of antigen
Indirect ELISA detects antibody: AG is bound to the plate, an unlabeled primary antibody binds to it, followed by a labeled secondary antibody that recognizes the primary antibody. Slightly more steps, more flexible, more sensitive. Detects low amts of antibody levels.
What are Monoclonal Antibodies?
Used in ELISA test.
Mice is injected with a foreign AG
It’s B cells produce antibodies to recognize the antigen
7 days later - antibody producing B cells are taken from mice’s spleen + pure tumor cells (harvested from culture)
Mice B. Cells and tumor cells are mixed together - forming hybridoma cells
Only the fused hybridomas are grown in a selective medium
These successful hybridomas are screened for antibody production. They are then cultured to produce large number of MONOCLONAL antibodies.
What is Neutralization test?
It tests effectiveness of antibodies that are made in the lab - can they bind to the toxin and prevent it from damaging the cell or not. It blocks the harmful effects of a bacterial toxin or virus. Positive test = antigen was neutralized
Can also be used tested to see if virus + antibodies can bind together and neutralize the virs.