Elements & Periodic Table - A0S 1 - Chapter 2
1/46
Earn XP
Description and Tags
Remembering - Definitions
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
47 Terms
Atom
Smallest building block of matter
John Dalton’s Model
“An atom is comprised of many tiny spherical particles which are indestructible and indivisible.” - Deduced in 1802
Elements
Atoms containing only one type of atom
Compound
Atoms containing many different elements
Protons, Neutrons, Electrons
Positively charged, neutrally charged and negatively charged particles within an atom.
Size of electron
8000 times smaller than a a proton
Electrostatic attraction
Positive electrons attracting negative electrons
Nucleons
Protons & Neutrons
Flame Test (Purpose)
To distinguish metallic elements when put in a flame.
Order of Energy Emitted In Terms Of Color
Violet, blue, green, yellow, orange, red
Bohr Model
“Electrons move around in fixed orbits around the nucleus, electrons have fixed energy levels, electrons cannot exist between two energy levels, larger orbits correspond with larger energy levels.” - Deduced in 1913, by Niels Bohr
Electron Shells
Energy levels where electrons are grouped in.
Effects Of Heating An Element
Electron jumps to a higher energy state, and the energy is then emitted by light or heat, as the electron returns back to its original state.
Ground State
Lowest energy level for an atom
Excited Energy State
Electron when “excited”.
Formula for no. of electrons per shell
2n²
Problems With Bohr Model
Unable to explain why shells can only hold 2n² electrons
Can’t predict emission spectra for atoms that have more than one electron
Can’t explain why 4th shell gain 2 electrons before 3rd shell fills up.
New Concept In the Schrodinger Model Vs. Bohr Model
Concept of “orbitals” and “subshells'“
Subshells & Oribitals
Subshells: Shells that contain separate energy levels (s,p,d,f)
Orbitals: Smaller components within the subshell.
Electrons To Be Held In An Orbital (Max)
2
Orbitals present in s,p,d,f
1,3,5,7 (possible electrons being: 2,6,10,14)
Energy order of subshells
1s,2s,2p,3s,3p,4s,3d
Exceptions For Rule Of Orbitals (Elements)
Chromium, Copper
Electron Configuration for Chromium & Copper
Chromium: 1s²,2s²,2p^6,3s²,3p^6,3d^5,4s1
Copper: 1s², 2s², 2p^6, 3s², 3p^6, 3d^10, 4s^1
Reason For Copper & Chromium Electron Arrangement
It is slightly more stable in this configuration.
Main Group Elements In Periodic Table
Group 1,2 13-18 (Excluding Transition Metals)
Reason For Elements Having Similar Properties In Same Group
Have same no. of electrons
Properties Of Alkali Metals
Soft and reactive with oxygen and water.
Properties Of Noble Gases
Low reactive
Have a full Outer shell
Information stated by periodic arrangement
Number of shells, and is in the same column as other elements with same no. of shells
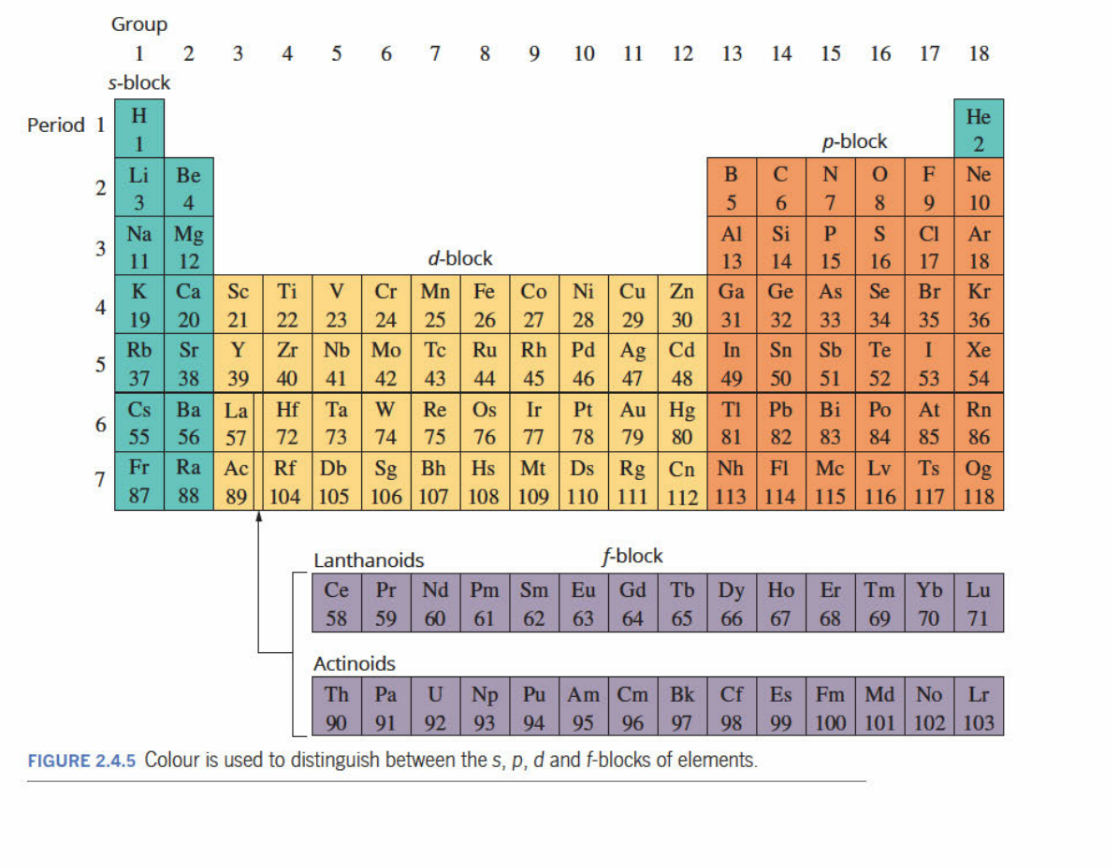
Blocks Within Periodic Table
These blocks indicate which elements have their valence electron in what subhell. Eg. “s” blocks elements have their valence electrons in the ‘s’ block.
Electronegativity
Ability for an atom to attract an electron (higher rating corresponds to higher ability)
First Ionization Energy
Energy required to remove one electron from the atom.
Person who observed periodic trends
Dimitrivi Mendeleev - 1869
Reason For Electrostatic Attraction Decreasing Down A Group
More shells, less electrostatic attraction retaining them together.
Effective Nuclear Charge/Core Charge
“Attraction felt by the valence electrons from the nucleus.”
Found by: no. of valence electrons
Core Charge Across A Period
Increases - due to more valence electrons
Electronegativity - down the group and across the period
Decreases Down Group: Electrons become more distant from nucleus despite core charge remaining the same.
Increases Across Period: Number of valence electrons increase, thus increasing core charge - increasing electrongeativity.
Atomic Radius - down group and across period
Increases down the group: More shells thus higher atomic radius.
Decreases down the period: More valence electrons - more electrostatic force - less atomic radius.
First Ionization Energy - Across Period, Down Group
Across Period - Increases: More electrostatic force holding together electrons due to higher core charge.
Down Group - Decreases: Less electrostatic force, as more shells are being formed and less electrostatic attraction.
Most Reactive Metals
Group 1 - Alkali Metals
Properties For Metals Down A Group
More Reactive - More Shells, and less core charge and electrostatic attraction. Electrons can easily be lost without a lot of ionization energy - thus more reactive.
Non-metal electron gaining properties
Across Period: More reactive
Down Group: Less reactive as they gain electrons unlike metals.
Most Reactive Metal
Francium
Most Reactive Non-Metal
Fluorine
Thermal Conductivity
The ability to transfer heat
Non-metals reactivity
Decreases down a group - harder to attract electrons down a group
Increase across the period