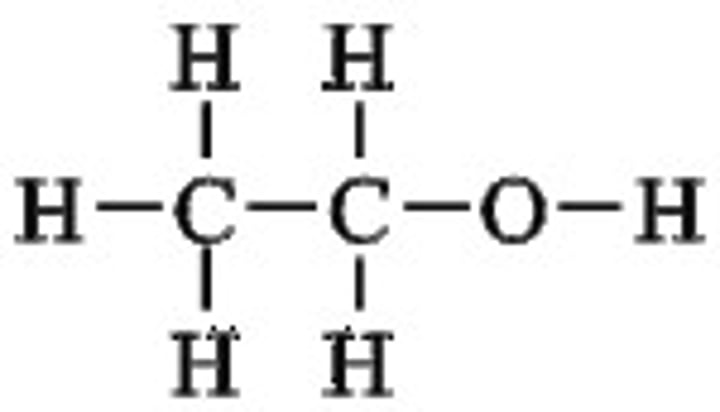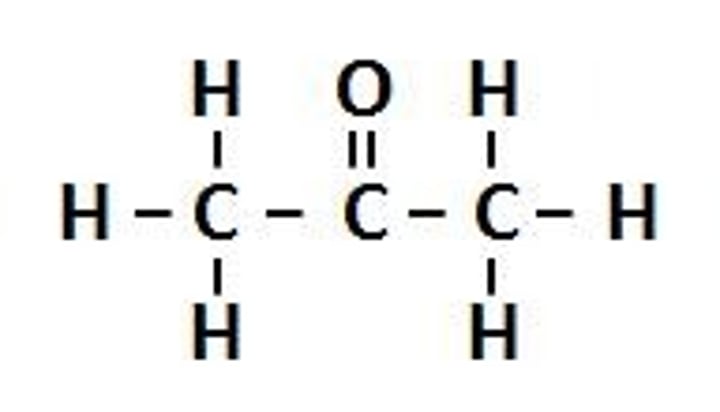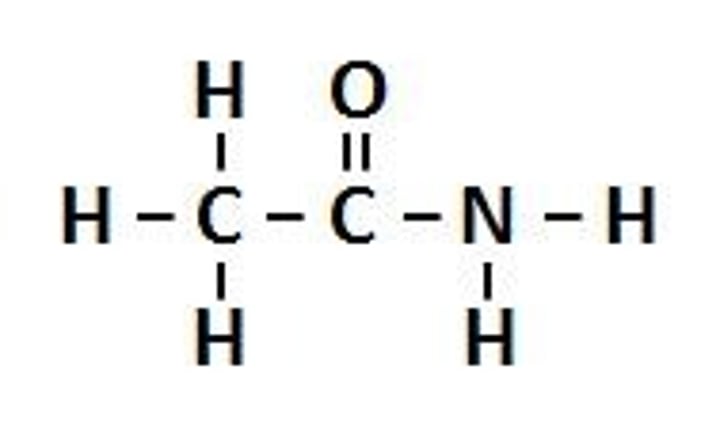Topic 10: organic chemistry
1/72
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
73 Terms
carbon
the chemistry of carbon is much more extensive than that of any other element with many compounds of carbon. the macromolecules that build up life are made of carbon
4 valence electrons, abundance and high bonding capacity
difference between O2 and O3
O2 is stronger than O3 and O2 absorbs more UV
Why does benzene undergo substitution more readily than addition?
resonance makes C_C bonds too strong to break easily
catenation
the fact that carbon atoms can join together to form chains and rings
fractional distillation
components in a chemical mixture are separated using their different boiling points.
Alkanes
a family of hydrocarbons
reactivity of alkanes, alkenes and alkynes
Alkanes are the least reactive, alkene is more reactive, and alkyne is the most reactive
Atom/group of atoms that give the molecule its chemical properties. They are the reactive part of the compound
Structures of straight/ branched chains/ rings of carbon atoms
Alkanes
Alkenes
Alkynes
Cyclic
Carbons bonded by single bonds (sigma bonds) making them fully saturated hydrocarbons. very stable. CnHn+2
A molecule that has at least one double bond (pi bond) between two carbon atoms, resulting in an unsaturated hydrocarbon (not the maximum number of hydrogens are bonded to the carbon atom). Unstable/reactive. CnH2n
A molecule that contains at least one triple bond between carbon atoms, wrestling in an unsaturated hydrocarbon. CnH2n-2
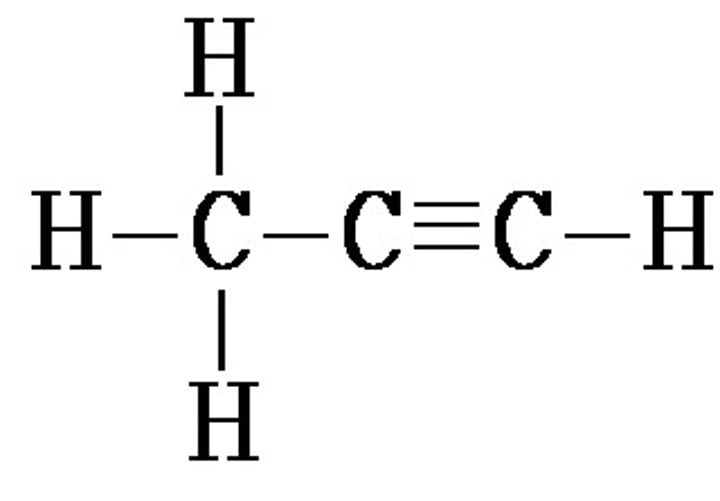
Structures based on that of benzene. Essentially the functional groups are the benzenes
A ring of 6 carbon atoms that are bonded to one another with double and single bonds with a resonance structure C6H6
-the electron in the double bond are shared equally among all the carbons
Very stable molecule because of its resonance
“Pi electrons are delocalized in its arrangement “
Benzene with a methyl (CH2) substituent group
Functional group: hydroxyl
Naming: anol
Example: Ethanol
General Formula: CnH2n+1OH
Form hydrogens bonds because of the hydroxyl group
Higher melting/boiling points since it has hydrogen bonds
o
: bonded to 1 carbon alkyl
Secondary Alcohol 2o: bonded to 2 carbon alkyl
Tertiary Alcohol 3
o
: bonded to 3 carbon alkyl
Aldehydes
Functional group: aldehydes (carbonyl)
Suffix: anal
Example: Ethanal
General formula: RCHO
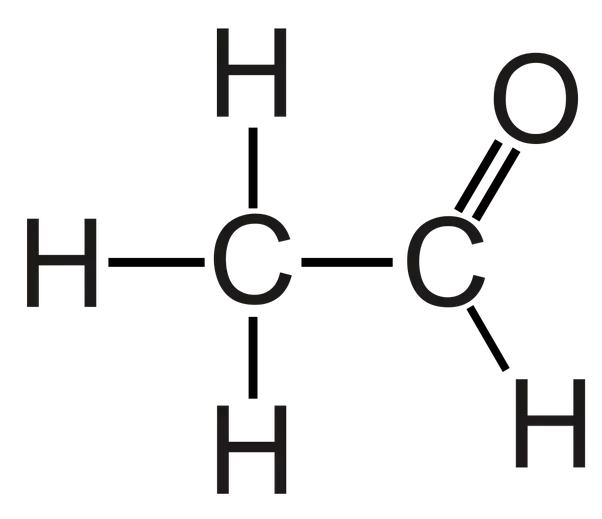
Also are less soluble in water than alcohols
However, C=O bond is polar so they are more soluble and have higher boiling and melting points than hydrocarbon
Functional Group: Carbonyl
Suffix: anone
Example: Propanone
General formula: RC(O)R'
However, C=O bond is polar so they are more soluble and have higher boiling and melting points than hydrocarbon
If it’s not the priority it’s represented with “oxy”
always as a prefix
More soluble and higher melting/boiling points than hydrocarbons
Less polar and lower melting/boiling points than alcohols
Main chain suffix end is “oate”
More soluble and have higher mp/bp point than ethers and hydrocarbons but less soluble and lower mp/bp than alcohols and carboxylic acids (as an aqueous solution)
Main chain suffix end is “amine”
Higher mp/bp than hydrocarbons but because it’s less polar than O-C groups it is lower than alcohols
o
: 1 hydrocarbon chain
Secondary Amine 2
o
: 2 hydrocarbon chains
Tertiary Amine 3
o
: 3 hydrocarbon chains
Main chain suffix end is “amide”
Higher mp/bp than ketones/aldehydes but a lower polarity than carboxylic acids
When maximum number of hydrogens are bonded to the amide it has a higher boiling point because it now has more hydrogen bond
Propagation
Termination
Splits the electron equally, both atoms get one of the previously shared electrons - Forming the free radicals with the divided atoms
Written as X•
Radical takes hydrogen from the hydrocarbon forming a free radical hydrocarbon
Then the free radical hydrocarbon reacts with an element (that was originally the free radical from the initial step) and forms a compound with it and there is now an extra free radical of the original free radical
Number of covalent bonds divided by the number of atoms involved in the compound
bond length which tells us how strong it is: Smaller bond lengths means it is a stronger bond and vice-versa
2
divides into two oxygen free radicals
Bond order of 2 → longer bond length → Weaker → less energy needed to break
Only needs UV-C
3
divides into 1 oxygen free radical and then an oxygen compound
Bond order of 1.5 -> Shorter bond length → stronger → more energy needed to break
Needs UV-B
destroys the ozone molecules when it is broken down by the UV light
Made up of monomers
Unreactive
Extremely reactive
Have many properties
Double bonds break and then a bond that is adjacent to the monomers form on BOTH sides of the original monomer
Polypropylene (made from a chain of propylene) ← Propene
Polyvinylchloride
Polystyrene (made from a chain styrene) ← Phenylethene
Teflon
Producing amides and esters and water
Condensation Reactions
, a condensation reaction is a type of chemical reaction in which two molecules are combined to form a single molecule, usually with the loss of a small molecule such as water. If water is lost, the reaction is also known as a dehydration synthesis
Functional group: hydroxyl Naming: anol Example: Ethanol General Formula: CnH2n+1OH
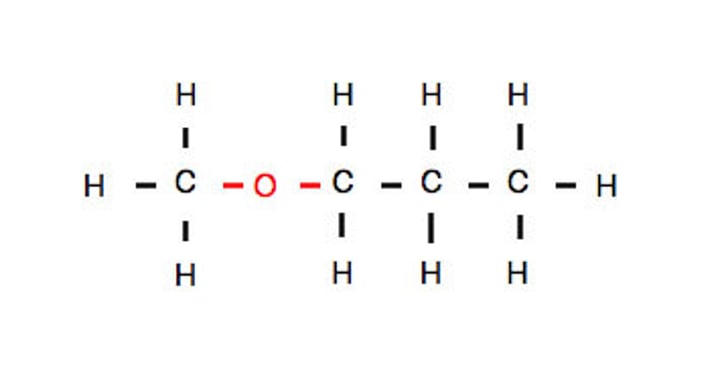
Functional group: ether Suffix: oxy alkane Example: Methoxy Propane General formula: ROR'
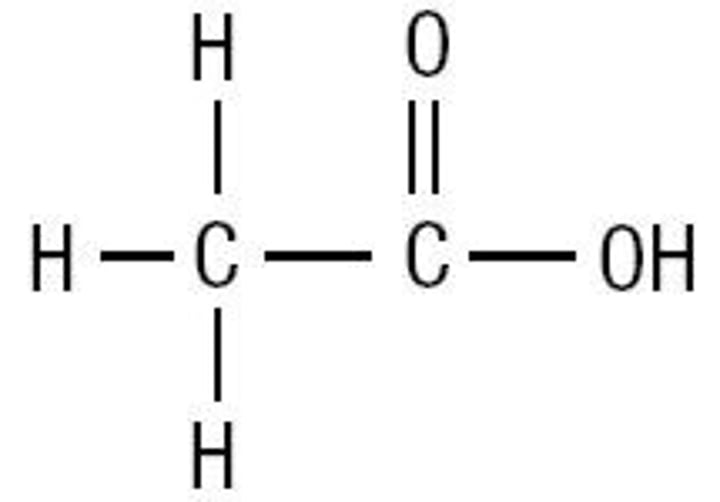
Functional group: Carboxylic acid Suffix: anoic acid. Example: Ethanoic Acid General formula: RCOOH. OH groups make these even more polar than other functional groups, so very soluble in water very high melting/boiling points
Suffix: alkyl anoate
Example: Methyl Propanoate
General formula: RCOOR'
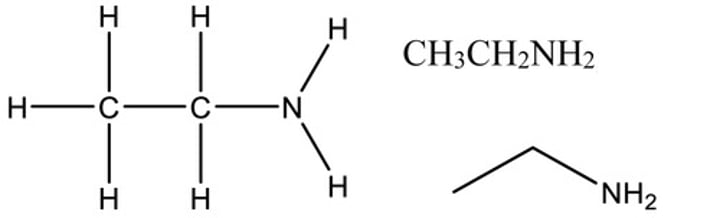
Functional group: Amine Suffix: anamine Example: Ethanamine General Formula: RNH2
Functional Group: Amine Suffix: anamide Example: Ethanamide General Formula: RC(O)NH2
alkene/alkyne colour change in bromine water
orange→ colourless
alkane colour change in bromine water
no colour change