Nuclear Chemistry and Molecule Shapes
0.0(0)
0.0(0)
Card Sorting
1/10
There's no tags or description
Looks like no tags are added yet.
Last updated 1:39 PM on 11/22/25
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
11 Terms
1
New cards
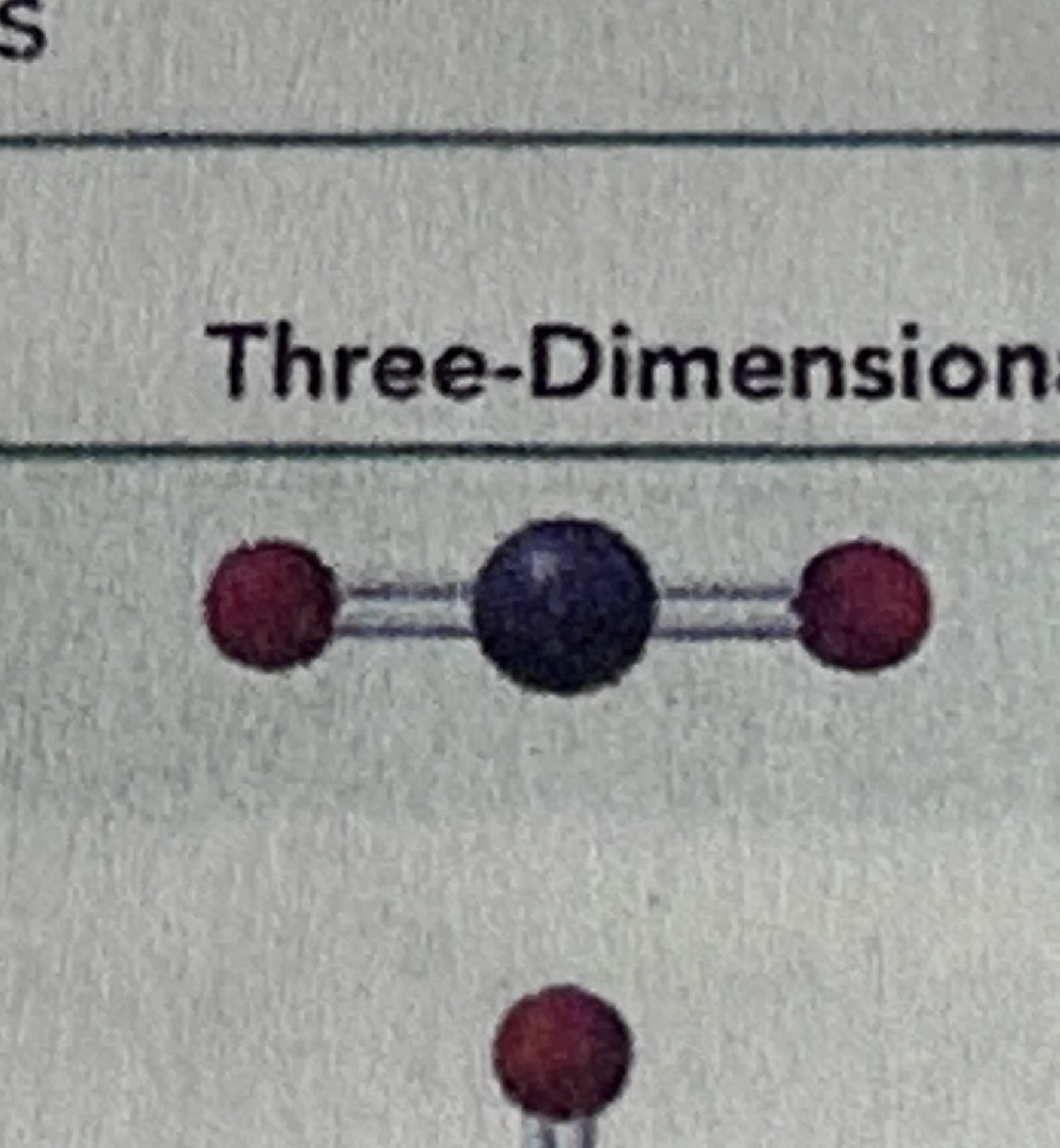
Linear has a bond angle of…
180 degrees
2
New cards
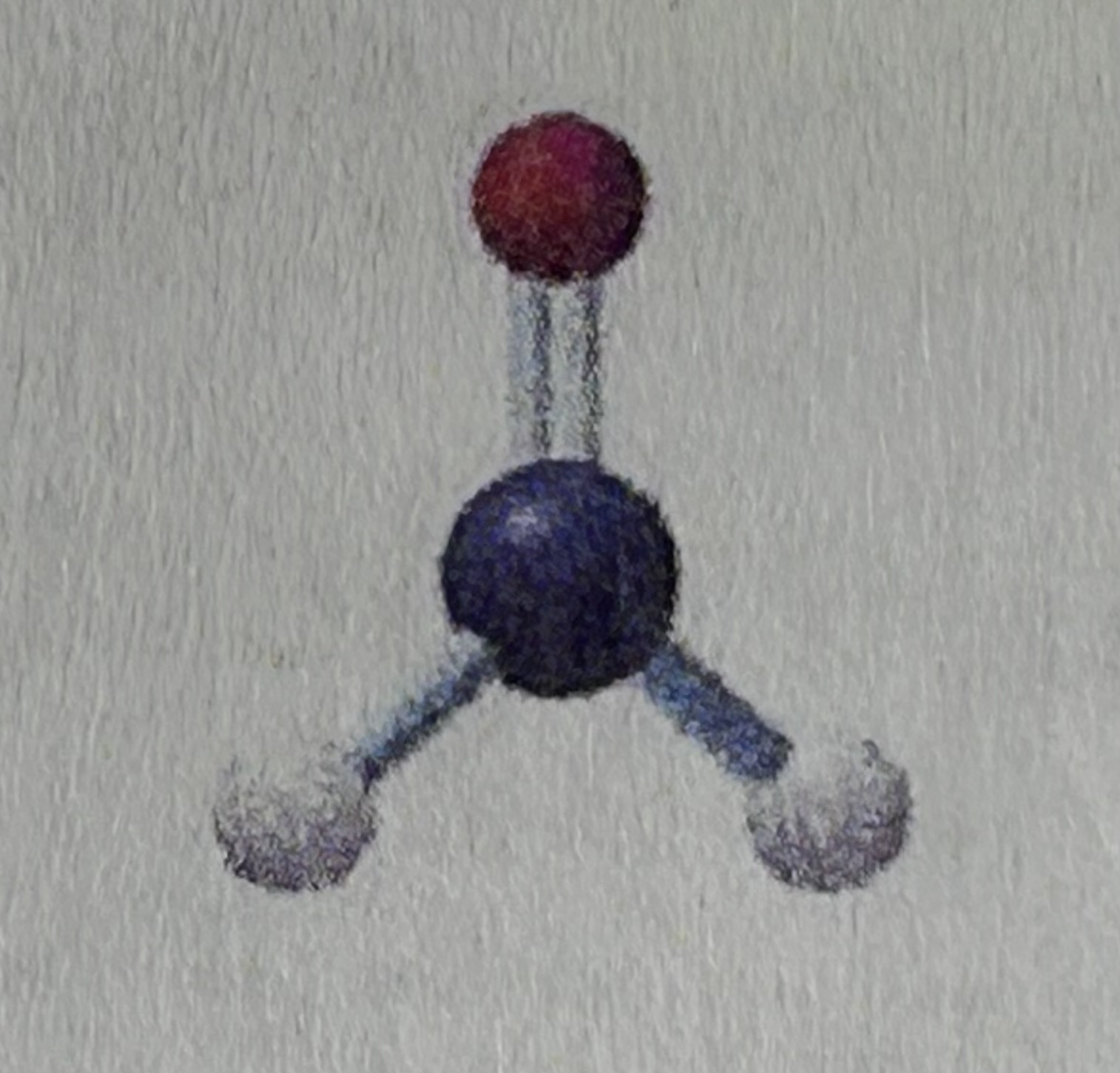
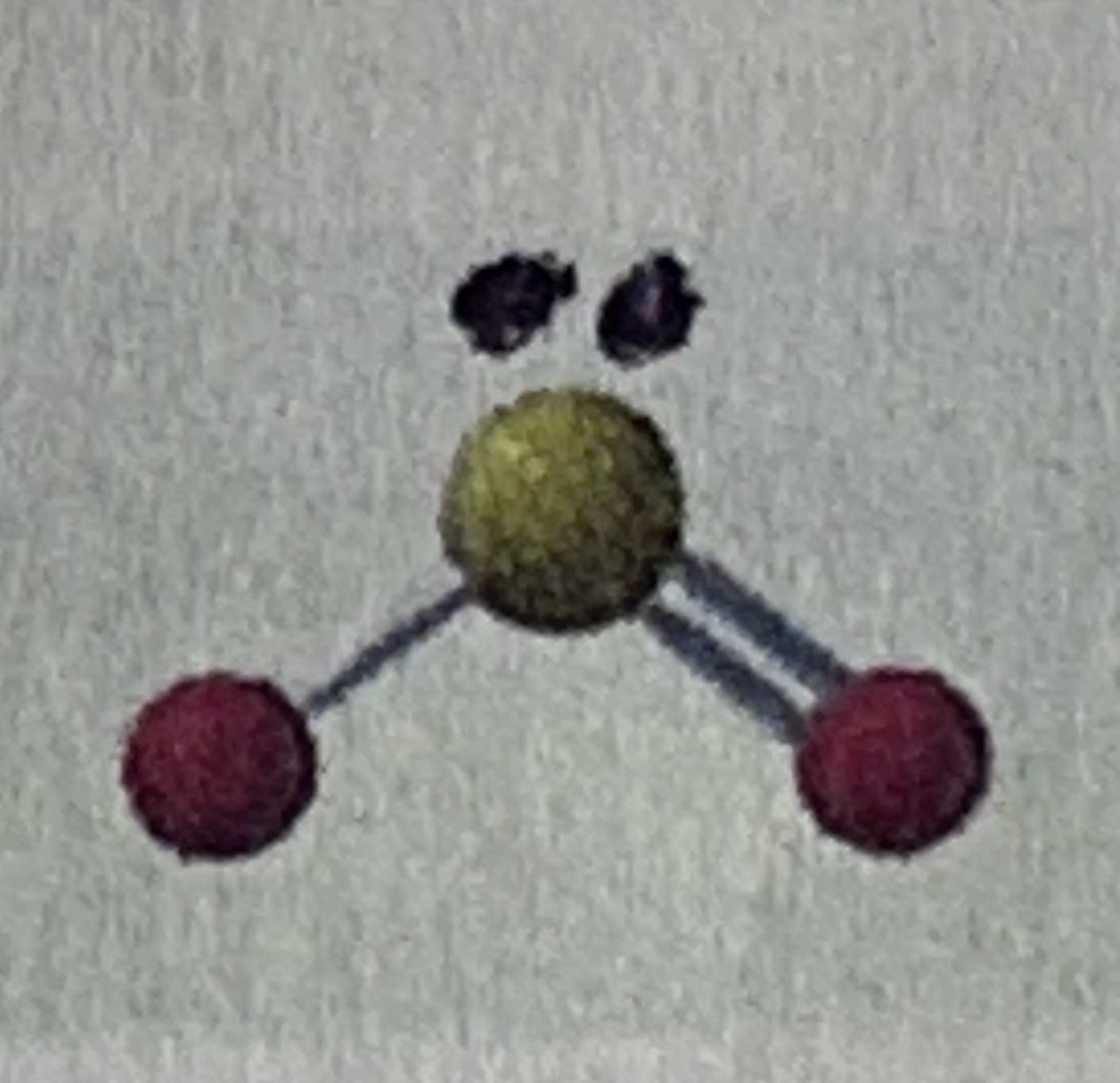
Trigonal planar has a bond angle of…
120 degrees
3
New cards
Bent (one lone pair) has a bond angle that is less than…
120 degrees
4
New cards

Tetrahedral has a bond angle of…
109 degrees
5
New cards
How many becquerels are in a curie?
3.7 × 10^10 Bq
6
New cards
7
New cards
8
New cards
9
New cards
LD stands for what?
Lethal dose
10
New cards
What does LD 50 mean?
The lethal dose that is expected to cause 50% of a population’s death.
11
New cards
What is the rem amount that causes LD 50 in humans?
500