Chapter 2: The First Law of Thermodynamics
1/99
Earn XP
Description and Tags
- energy - work - heat - 1st law - enthalpy - internal energy - heat capacities - changes in state functions for isotherm., ad., irr., & rev. processes
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
100 Terms
first law of thermodynamics
there is an extensive state function E (total energy of sys.) for any process in closed sys.
ΔE is energy change undergone by sys. in process
change in energy of sys. ΔE happens along change in energy of surroundings −ΔE
total energy of a system + surroundings remains constant (is conserved)
ΔU = Q + W (restrict to closed sys., at rest, no ext. fields)
E = U
what do the heat capacities at constant volume and pressure tell us?
they give the rates of change of the internal energy and enthalpy with temperature
what is 1 N
1 N = 1 kg*m/s2
what is the unit for work?
joules, J
what is a joule? 1J=?
1J = 1N*m = 1 kg*m2/s2
if F is the total force acting on a particle from point 1 to 2, how can u find the total work done?
the total work done will be from the integration of F*dx from point 1 to 2 in each direction summed up
how to calculate work using kinetic energy?
work is the change in kinetic energy on a system
w = K2 — K1
what does the work-energy theorem say?
the work done on a particle by a force equals the change in kinetic energy of the particle
dw is the infinitesimal work, to get the total amount of work, what do u do?
an infinite amount of infinitesimal are summed up to calculate integral
so integration is done
closed system + reversible process
what is the infinitesimal change in work?
what is the total work done on system?
dwrev = −P*dV
wrev = −∫(1 to 2)P*dV
for work in a closed system + reversible process, what is the sign of work for expansion and compression?
expansion: work is negative, -ve, w<0
contraction: work is positive, +ve, w>0
what is a reversible process?
idealization
process where system is always infinitesimally close to equilibrium
infinitesimal change in conditions can reverse process to restore system + surroundings to initial states
what is the work done due to a volume change called?
P-V work
the value of the line integral is the area under..?
the curve that plots P vs. V
what does the work depend on?
work depends on process used to go from state 1 to state 2
what will happen when 2 bodies at unequal temps. are placed in contact?
they will eventually reach thermal equilibrium at a common intermediate temp
conventionally, heat has flowed from hotter to colder body
specific heat capacities of substances are functions of what?
temperature and pressure, T and P
What does this equation: dqP = m*cP*dT; mean in words
in a closed system, when infinitesimal amount of heat dqP flows at constant pressure, P into a body of mass m and specific heat capacity at constant pressure cP the body’s temp is raised by dT
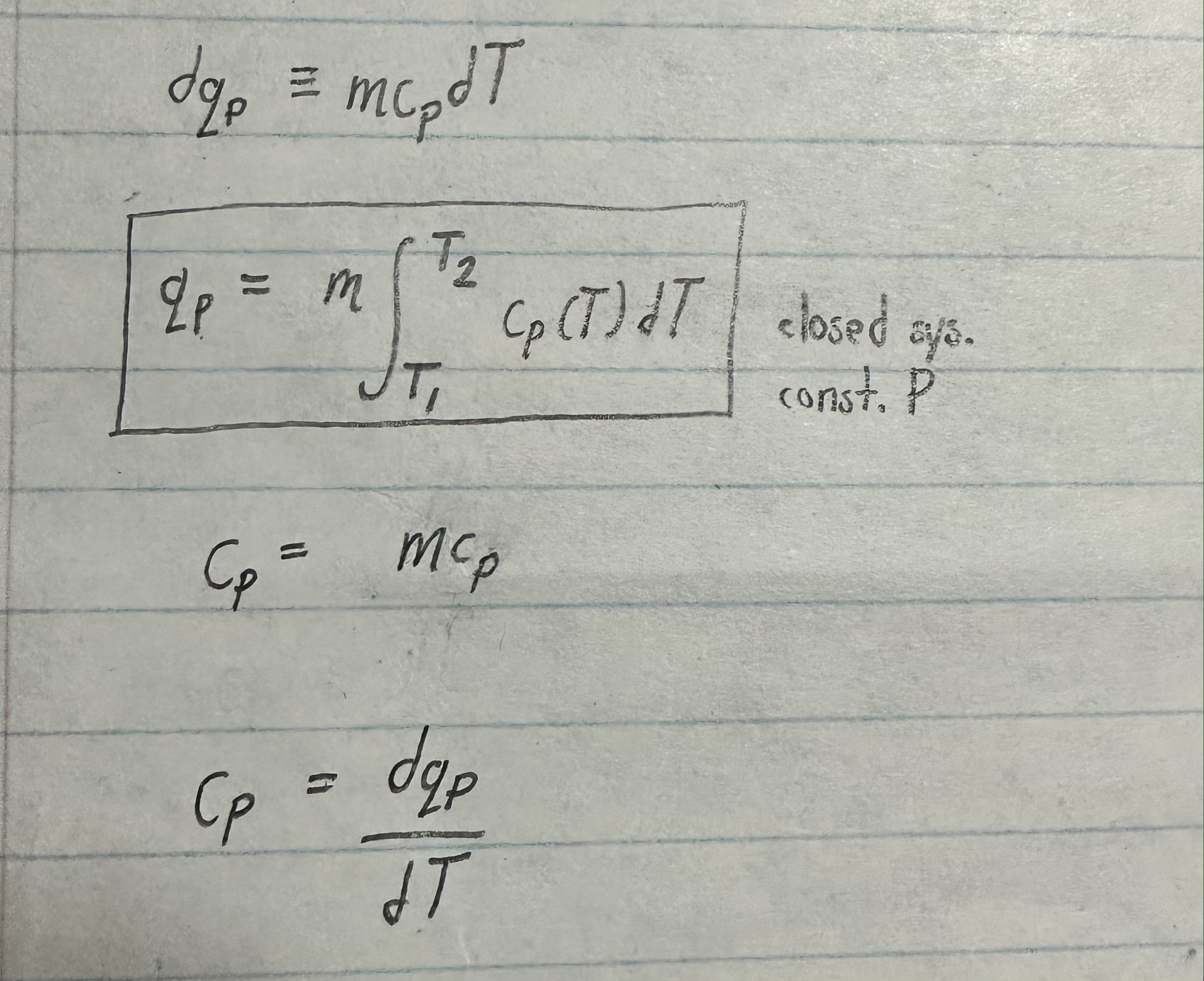
when is heat flow reversible or irreversible?
heat flow is reversible when the temperature difference btwn the bodies is infinitesimal
heat flow is irr. when there’s finite temp. difference btwn bodies
what is the internal energy, U?
internal energy is energy that exists at molecular level and includes molecular kinetic and potential energies
internal energy consists of molecular translation, rotational, vibrational, and electronic energies
molar internal energy symbol, calculation, units
Um = U/n
n = number of moles
J/mol
when is q negative or positive?
when heat flows into sys. from surroundings during a process, q>0
when outflow of heat from sys. to surrounds, q<0
what is the internal energy for a closed system for an infinitesimal process?
dU = dq + dw
U is a state function, what does this mean?
the change in U only depends on the final and initial states of the sys., its independent of path used to bring sys. from initial to final state
what is the process called when the final state of the system is the same as the initial state?
cyclic process, ΔU = 0
what are heat and work defined in terms of?
heat and work are NOT forms of energy
heat and work are forms of ENERGY TRANSFER
heat and work exist only in terms of processes
they don’t exist before or after process of energy transfer btwn sys. and surro.
heat is energy transfer btwn sys. and surro. due to temp difference
work is energy transfer btwn sys. and surro. due to macroscopic force acting thru distance
heat is work done at the _________ _______
molecular level
what is “heat flow” in actual thermodynamic terms?
“heat flow“ is energy flow due to temp diff.
units of heat
joule, J
for heat, 1 cal = ?J
1 cal = 4.184J
what is the equation for enthalpy
H = U + PV
units of enthalpy
joules, J
for P-V work, in a closed system, with constant pressure
what is the equation for a change in enthalpy? for what kind of changing work?
ΔH = qp
only for volume-change work
what is the enthalpy change for a change of state?
ΔH = U2 + P2V2 − (U1 + P1V1)
ΔH = ΔU + Δ(PV)
what is the enthalpy change for a constant pressure process?
P2 = P1 = P
Δ(PV) = PV2 − PV1
ΔH = ΔU + PΔV
Δ(PV) is different from … and …
P*ΔV and ΔP*V
because only the change in one term is taken
as opposed to the difference in PV altogether
molar enthalpy symbol, calculation, units
Hm = H/n = (U + PV)/n = Um + PVm
enthalpy is molar internal energy + pressure times molar volume
what is the first law for a constant-volume process
closed sys., P-V work only, V constant
ΔU = qv
qv is where heat absorbed at constant volume
what is the heat capacity of a closed system for an infinitesimal process?
Cpr = dqpr/dT
what does: Cpr = dqpr/dT mean in verbal terms?
it means the heat capacity is the heat flowing into the sys. and the temp. change of sys. in the process
the subscript means that heat capacity depends on the nature of the process
what is the formula for isobaric heat capacity of a closed system for a constant-pressure process?
CP = dqP/dT
the subscript indicates that pressure is constant
what is the formula for isochoric heat capacity of a closed system for a constant-pressure process?
CV = dqV/dT
the subscript indicates that volume is constant
what does: CV = dqV/dT mean in verbal terms?
it means the heat capacity of a closed system during an infinitesimal constant-volume process.
it refers to the heat flowing in the sys. and the temp change of the sys. during process
what kind of process do formulas for heat capacity of closed system work for?
CV = dqV/dT
Cpr = dqpr/dT
they work for reversible processes
for an infinitesimal process:
dqP = dH at const. P
dqV = dU at const. V
so what can the formula for heat capacities be written as for a closed system in equilibrium, for P-V work?
CV = (dU/dT)V
CP = (dH/dT)P
what do these equations directly tell us (rate of change) ?
CV = (dU/dT)V
CP = (dH/dT)P
they give us the rates of change of H and U with temperature
any state function has a definite value once what is specified?
one the system’s state is specified
for a closed system of fixed composition, the state is specified by which functions/variables?
the state is specified by the properties and functions of P and T
any state function of a closed equilibrium system of fixed composition is a function of what?
T and P
what is CP a function of?
CP is a function of T and P
what is H (enthalpy) a function of?
H is a function of T and P
what is U (internal energy) a function of?
T and V
what is CV a function of?
CV is a function of of T and V
in “specific heat capacity”, “specific volume” and “specific enthalpy”, what does specific refer to? and how to obtain these values?
specific means “divided by mass”
cP, v, and h are obtained by dividing CP, V, and H by m
which of these are intensive or extensive properties?
cP
CP
CP,m
cP = specific heat capacity, intensive
CP = heat capacity, extensive
CP,m = molar heat capacity, intensive
for a closed system CP and CV must be positive/negative?
both must be positive
CP,m = CP/n
what is the formula for? and for what kind of sys.?
molar heat capacity for a pure substance
cP = CP/m
what is the formula for? and for what kind of sys.?
specific heat capacity for one-phase system
(dU/dV)T
what type of function is it?
what is it sometimes called?
how does it relate internal energy and volume?
it is a state function
sometimes called the internal pressure
relates to part of internal energy that occurs bc of intermolecular potential energy
change in system’s volume will change average intermolecular distance and thus average intermolecular potential energy
how do gases, liquids, solids and pressure relate to (dU/dV)T and whether it is big or small?
for gas, at low pressure, intermolecular forces are smaller
this makes (dU/dV)T smaller
for liquids and solids, pressure is higher, molecules are closer to e/o
intermolecular forces are larger
this makes (dU/dV)T larger
what is the sign of work when:
surroundings do work on system
system does work on surroundings
w>0, positive
w<0, negative
what is enthalpy as a concept, in words?
enthalpy is a measurement of energy in a thermodynamic system
enthalpy is the sum of internal energy and the product of pressure and volume of sys.
The Joule and Joule–Thomson experiments measure (dT/dV)U and (dT/dP)H; what are these derivatives closely related to?
closely related to (dU/dV)T and (dH/dP)T
switch the top(numerator) of derivative with subscript to find close relations
what does a perfect/ideal gas obey?
PV = nRT
(dU/dV)T = 0 (internal pressure)
(dH/dP)T = 0
U, H, Cv, CP depend only on T
CP - CV = nR
what did the joule experiment demonstrate for real + ideal gas?
the internal energy only depends on temperature T, not volume V
what processes are the changes in thermodynamic properties for ideal gas calculated for easily?
reversible isothermal
reversible adiabatic
line integral L∫(1 to 2)db is independent of path from state 1 to state 2 if …?
if b is a state function
if ∮db = 0 for every cyclic process, then b is a state function, the line integral, ∮db = 0 for what type of process?
cyclic process
the heat capacities are measures of how much _______ must be added to substance to give a certain _________ in __________
energy must be added to substance to give a certain increase in temperature
the more ways (translation, rotation, intermolecular interactions) a substance has of absorbing added energy, what will happen to its molar heat capacity values?
the greater its heat capacities with constant pressure and volume will be
what happens during the joule experiment? how is work calculated? what is the joule coefficient?
it’s abt measuring the T change after free expansion of gas into a vacuum
first chamber A has gas, during equilibrium, the temp is measured by thermometer
system has adiabatic walls, q = 0, no heat flows in/out
expansion into vacuum is irreversible
finite unbalanced forces act within system + lack of pressure equilibrium
dw = -PdV doesn’t apply, only motion is within system
gas do no work on surro., surro. do no work on gas
w = 0 for free expansion into vacuum
calculate work for closed system, ΔU = q + w
ΔU = q + w = 0 + 0, constant-energy process
experiment measure T change w change in V at constant U
joule coefficient: μJ = (dT/dV)U
experiment gave μJ = 0 = (dT/dV)U, improved version was done = JT experiment
how is μJ = (dT/dV)U is related to (dU/dV)T ?
the variables in partial derivatives r same; T, U, V
use property (dT/dU)V * (dU/dV)T * (dV/dT)U = −1
(dU/dV)T = −CV*μJ
what was the joule-thompson experiment? how was work calculated?
version of joule experiment to get more accurate results
involved slow pushing of gas through rigid, somewhat permeable plug
system was closed in adiabatic walls
left piston had fixed P = P1, right piston had fixed P = P2 < P1
want to calculate work done on gas by throttling through plug
process is irr. bc P1 > P2 by finite amount and infinitesimal change in P can’t reverse process
but P drop occurs almost only in plus
plus is rigid, gas does no work on plug + vice versa
exchange of work btwn sys. and surro. happens mostly at 2 pistons
P eq. is maintained at piston, can use dw = −PdV to. get work at each piston
initial + final volume of left chamber: V1, 0
right chamber: 0, V2
wleft = −∫(V1 to 0)P1dV = −P1∫(V1 to 0)dV = −P1(0 − V1) = P1V1
wright = −∫(0 to V2)P2dV = −P2∫(0 to V2)dV = −P1(V2 − 0) = −P2V2
gas in right chamber does work on piston = negative work
total work done, w = wleft + wright = P1V1 − P2V2
for gases, (dU/dV)T is nonzero but its _____
small
how was the change in enthalpy calculated for a JT experiment?
first law for adiabatic process, q=0, due to walls
ΔU = q + w = w, ΔU = P1V1 − P2V2 , U2 − U1= P1V1 − P2V2
rearrange to get U2 + P2V2= U1 + P1V1
H = U + PV, so H2 = H1, ΔH = 0
how is the joule-thompson coefficient defined?
μJT = (dT/dP)H
μJT is ratio of infinitesimal changes in 2 intensive properties, therefore, it’s an intensive property anf function of T and P
measurement of T change in JT experiment gives ΔT/ΔP at constant H
what are the conditions for a gas to be cooled by a JT expansion? what is sign of the pressure change over JT expansion
the conditions are that the JT coefficient must be positive over range of T and P involved
pressure change is less than 0, pressure would be decreasing
ΔP < 0, μJ > 0
in a J-T expansion, the initial and final enthalpies are?
the same/equal
what are values for (dT/dP)H found for J-T expansion?
start with initial P1 and T1
pick P2 less than P1
plot points (T1, P1) and (T1, P2)
repeat with same initial point (T1, P1), and different final pressures
leads to multiple points that are for states of equal enthalpy
join points to make curves
slope of curve at any point gives (dT/dP)H for the T and P at that point
what is an ideal gas?
an ideal gas has no intermolecular forces
the molecules occupy negligible space
why do we expect that for an ideal gas, U will not change with V at constant T and (dU/dV)T = 0?
if change V while holding T constant, average distance btwn mlcls changes but intermolecular forces are 0, so this will not affect U
average translational kinetic energy of gas mlcls is function of T only, will not change with volume, V
for closed sys. in equilibrium, internal energy (+ any state function) is expressed as function of T and V. but for perfect/ideal gas, U is independent of ____, so U = ?
U is independent of V
U = U(T)
for perfect/ideal gas, U is independent of V, so CV = (dU/dT)V becomes:
CV = ?, dU = ?
CV = dU/dT
dU = CV*dT
CV of a perfect/ideal gas depends only on T, so CV = ?
CV = CV(T)
using eq. for ideal gases, for perfect gas, H = U + PV = ?
H = U + nRT
for an ideal gas, enthalpy only depends on ?, H = ?
enthalpy only depends on T, H = H(T)
for perfect/ideal gas, H is independent of P, so CP = (dH/dT)P becomes:
CP = ?
CP = dH/dT
CP = CP(T)
for a perfect/ideal gas, PV = nRT, so (dV/dT)P = ?
(dV/dT)P = nR/P
for an ideal gas:
(dV/dT)P = nR/P
CP - CV = P((dV/dT)P
so CP - CV = ?, CP,m - CV,m = ?
CP - CV = nR
CP,m - CV,m = R
how to calculate work for a reversible isothermal process in a perfect/ideal gas, fixed amount of perfect gas?
isothermal = constant T process
perfect/ideal gas: PV = nRT
U = U(T)
ΔU = 0 for isothermal change of state, ΔT = 0
ΔU = q + w, 0 = q + w
q = −w, w = −q
dwrev = −PdV, dwrev = −(nRT/V)*dV
w = −∫(1 to 2)(nRT/V)*dV
= −nRT*∫(1 to 2)(1/V)*dV
if there was no 1/V, it would just be (V2 − V1)
since 1/V is there, use ln
w = −nRT(lnV2 − lnV1)
w = −nRT*ln(V1/V2) = −nRT*ln(P2/P1)
cuz PV = nRT, P and V are proportional, but opposite
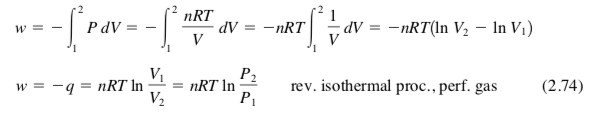
for a reversible isothermal process in a perfect/ideal gas, fixed amount of perfect gas
if the process is an expansion, what is the sign of w, q? and what does it rep.?
expansion, V2 > V1
w<0, negative, work done on system
q>0, positive, heat added to gas
describe in words what will happen for reversible isothermal process in perfect gas. using visual of gas in cylinder with const. T bath, with frictionless piston.
how is the external pressure changed?
what happens when pressure is increased or decreased?
the external pressure is changed at an infinitesimal rate
when the pressure is increased
gas is compressed
work is done on it, energy transfer to gas
temp. increases at infinitesimal rate
excess energy leaves as heat flows out of gas to const. T bath
maintains const. T for gas
when the pressure is decreased
the gas expands
gas does work on surro., energy transfer to surro.
temp. decreases
causes heat to flow into gas from bath
maintains const. T in gas
how to calculate change in internal energy for a reversible adiabatic process in a perfect/ideal gas?
adiabatic, dq = 0, q = 0
reversible process in sys. w/ only P-V work, dw = = −PdV
dU = CVdT, dU = dq + dw, ΔU = q + w = w
CVdT = −PdV = −(nRT/V)dV
CV,mdT = −(RT/V)dV
(CV,m/T)dT = −(R/V)dV = −∫(1 to 2)(R/V)*dV = R ln(V1/V2)
adiabatic, dq = 0
dU = CVdT
dU = dq + dw
ΔU = q + w = w
ΔU = CV(T2 − T1)
what is dU for an ideal gas for any process (no matter const. P, const. V, or not)?
dU = CVdT
what is dH for an ideal gas for any process (no matter const. P, const. V, or not)
dH = CPdT
for an ideal gas, CV,m is a function of T, if T change is small, CV,m can be taken as..?
approx constant
CV,m is nearly constant for what type of gas?
monatomic gases
for a perfect/ideal gas, adiabatic process, with CV constant, what is the relation ship between T1, V1, T2, V2, R, CV,m ?
(T2/T1) = (V1/V2)^ R/CV,m
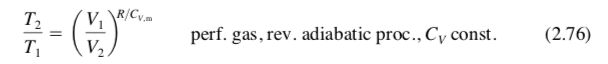
what is the heat capacity ratio gamma γ equation?
γ = CP/CV
if (contour integral) ∮db = 0 for every cyclic process, what is b?
b is a state function