Is My Product a Medical Device?
1/14
Earn XP
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
15 Terms
Where is the definition of a medical device defined in FDA
Section 201(h) of the Food, Drug & Cosmetic Act (FD&C Act)
Definition of a Medical Device
recognized in the official National Formulary, or the United States Pharmacopoeia, or any supplement to them
diagnosis, or in the cure, mitigation, treatment, or prevention of disease in man or other animals
intended to affect the structure or any function of the body of man or other animals
primary purpose not achieved through chemical action and not dependent on metabolization to achieve purpose
does not include software functions excluded pursuant to section 520(o).
Examples of Excluded Software
Administrative support of health care facility;
Maintaining or encouraging a healthy lifestyle unrelate
electronic patient records
Transferring, storing, converting formats, or displaying test or results but not intended to interpret or analyze them.
Know Your Product
• What is the intended use of your product?
• How does your product function?
• What claims do you intend to make?
Defining Your Intended Use is Key!
Clearly state the general purpose or function
disease or condition of intended use
intended patient population
Special Considerations: In Vitro Diagnostics (IVDs)
Reagents, instruments, and systems intended for use in the diagnosis of disease or other conditions.
Collect, prepare, and examine specimens
laboratory, health professional setting, or at home
• Examples: Home Pregnancy Test, Glucose Test Strip
Radiation Emitting Products
Section 531 of the FD&C Act:
product which when in operation
contains or acts as part of an electronic circuit
emits electronic product radiation
Most radiation-emitting products are not medical devices
Examples: Diagnostic Ultrasound, X-Rays, Medical Lasers
Mobile Medical Applications
(MMAs) in middle
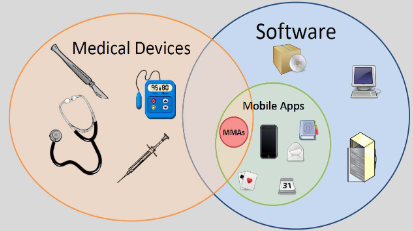
Software as a Medical Device (SaMD)
software intended to be used for one or more medical purposes that perform these purposes without being part of a hardware medical device
Software that allows a smartphone to view images obtained from a magnetic resonance imaging (MRI) medical device for diagnostic purposes
General Wellness Products
Products must meet the following two factors:
1. Are intended for only general wellness use, as defined in the guidance, and
2. Present a very low risk to users’ safety
Combination Products
therapeutic and diagnostic products that combine drugs, devices, and/or biological products
Lead center is based on a determination of the “primary mode of action” (PMOA)
Examples: Drug Eluting Stent, Heparin Coated Dialysis Catheter, First-Aid Kit with a Drug
Products Regulated by Other FDA Centers
Center for Drug Evaluation and Research (CDER)
Center for Biologics Evaluation and Research (CBER)
Center for Veterinary Medicine (CVM)
Center for Tobacco Products (CTP)
Medical Device Definition Questions
1 Is it intended to diagnose, cure, mitigate, treat, or prevent disease in a human?
2 Is it intended to affect the structure or any function of the body?
3 Does it achieve its primary intended purpose by chemical action or by being metabolized?
Additional Questions
Is there an existing product classification?
Is the product regulated as a medical device?
Summary
• Medical devices are defined under Section 201(h) of the FD&C Act
• A clearly defined intended use is key
• Identifying an existing medical device product classification can be helpful
• Consider further assistance if necessary