VSEPR
1/18
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
19 Terms
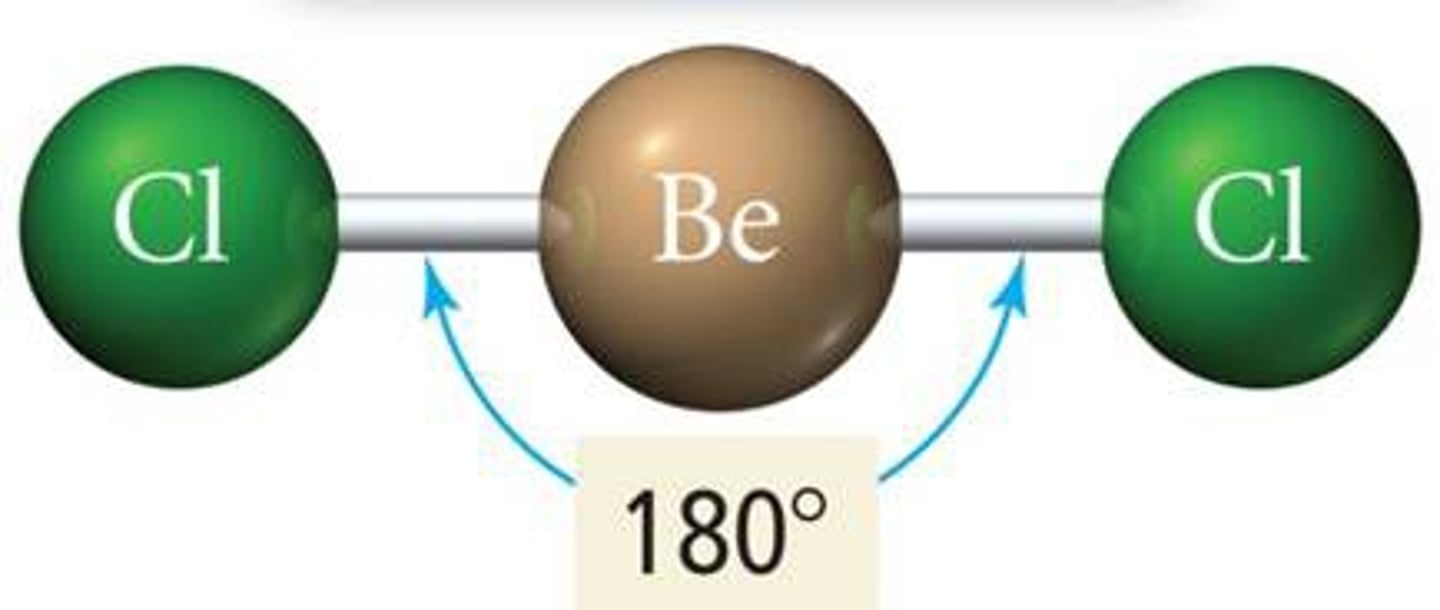
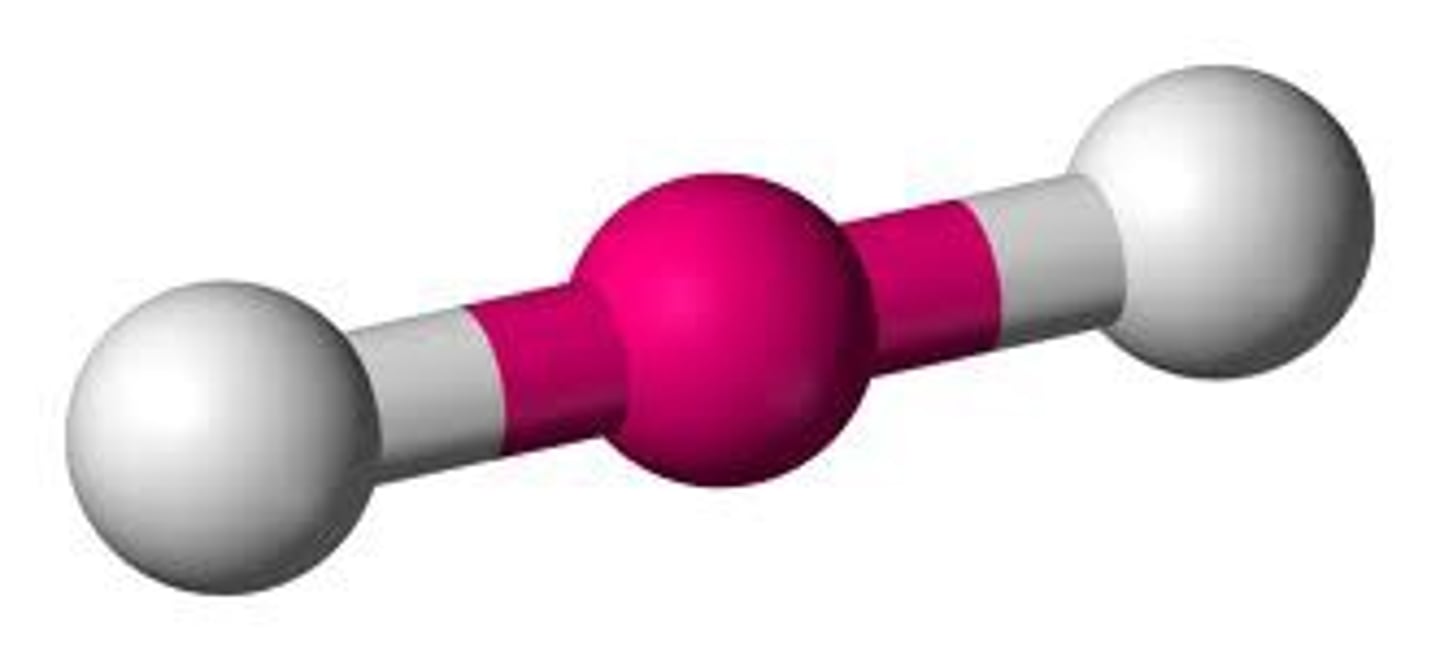
2 groups around central atom
linear (180)
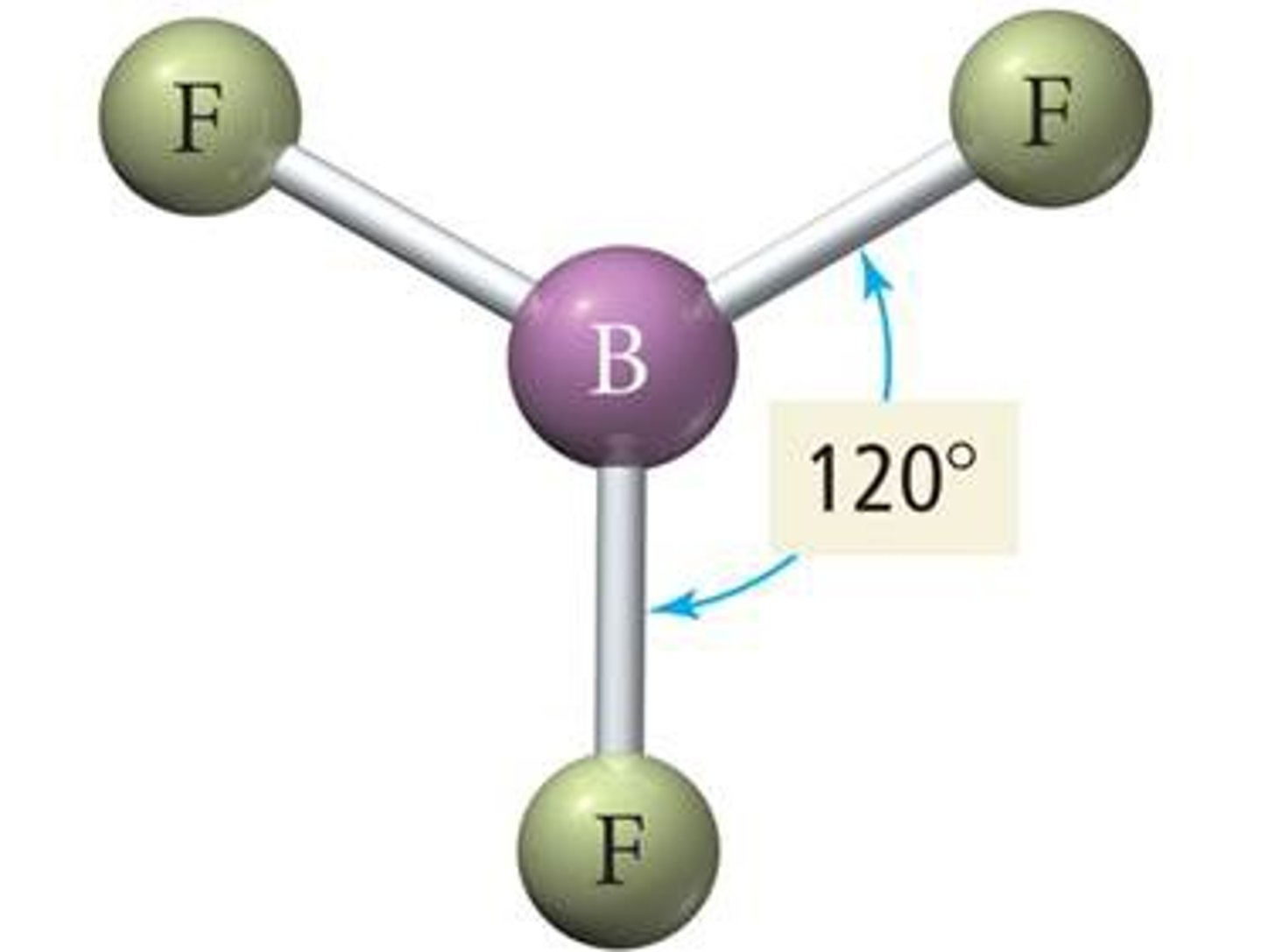
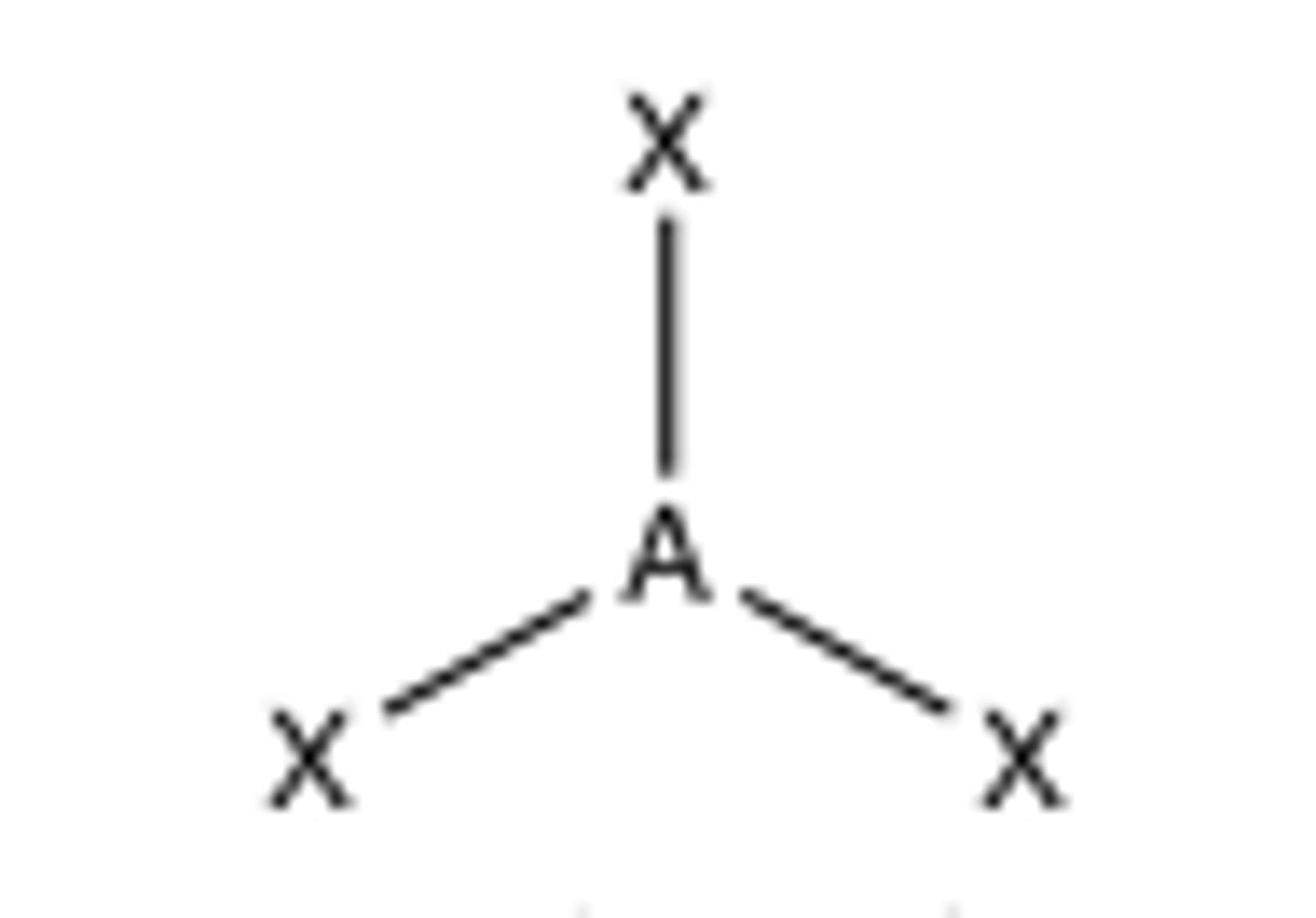
3 groups around central atom
trigonal planar (120)
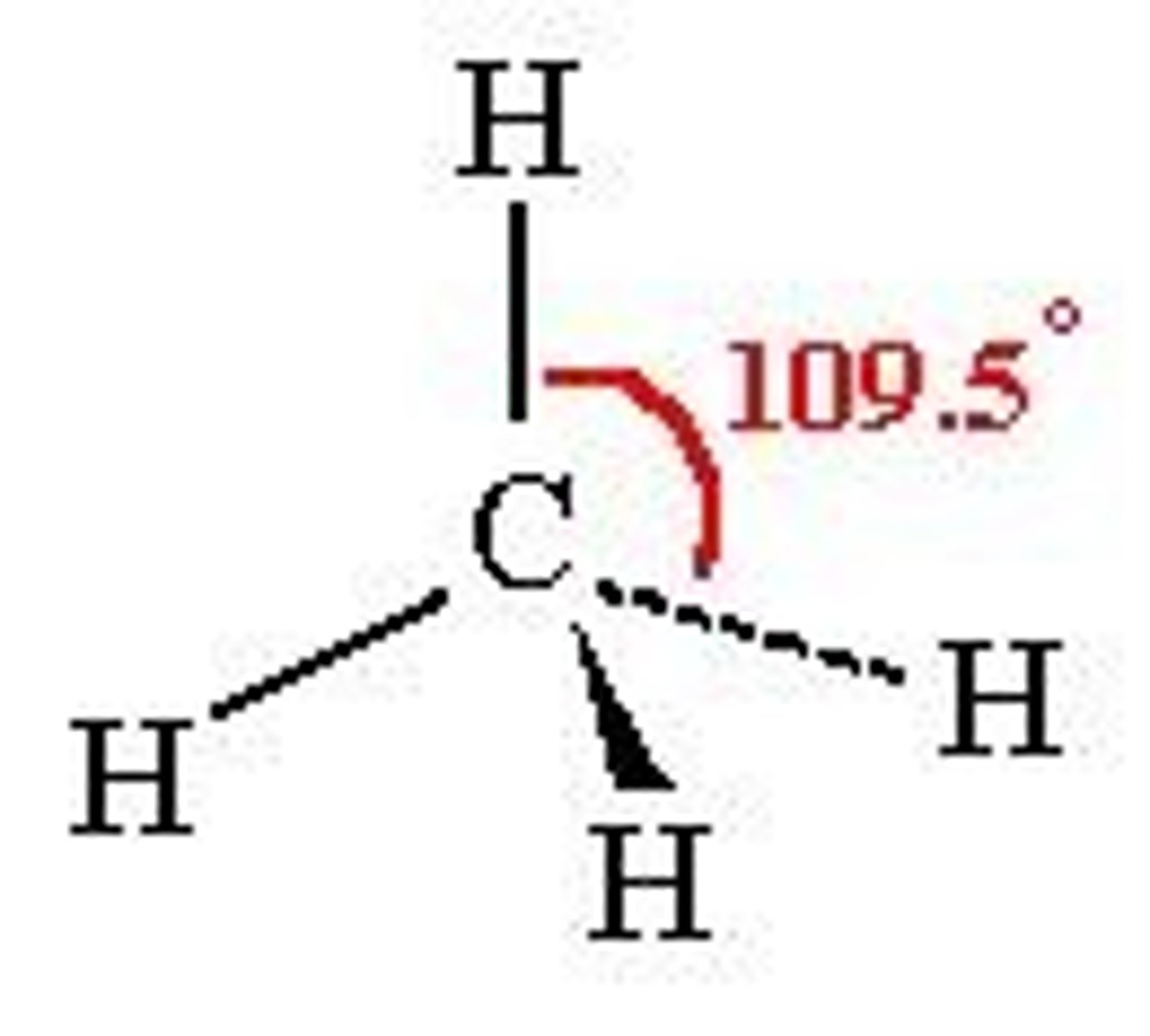
4 groups around the central atom
tetrahedral (109.5)
5 groups around central atom
trigonal bipyramidal (90, 120)
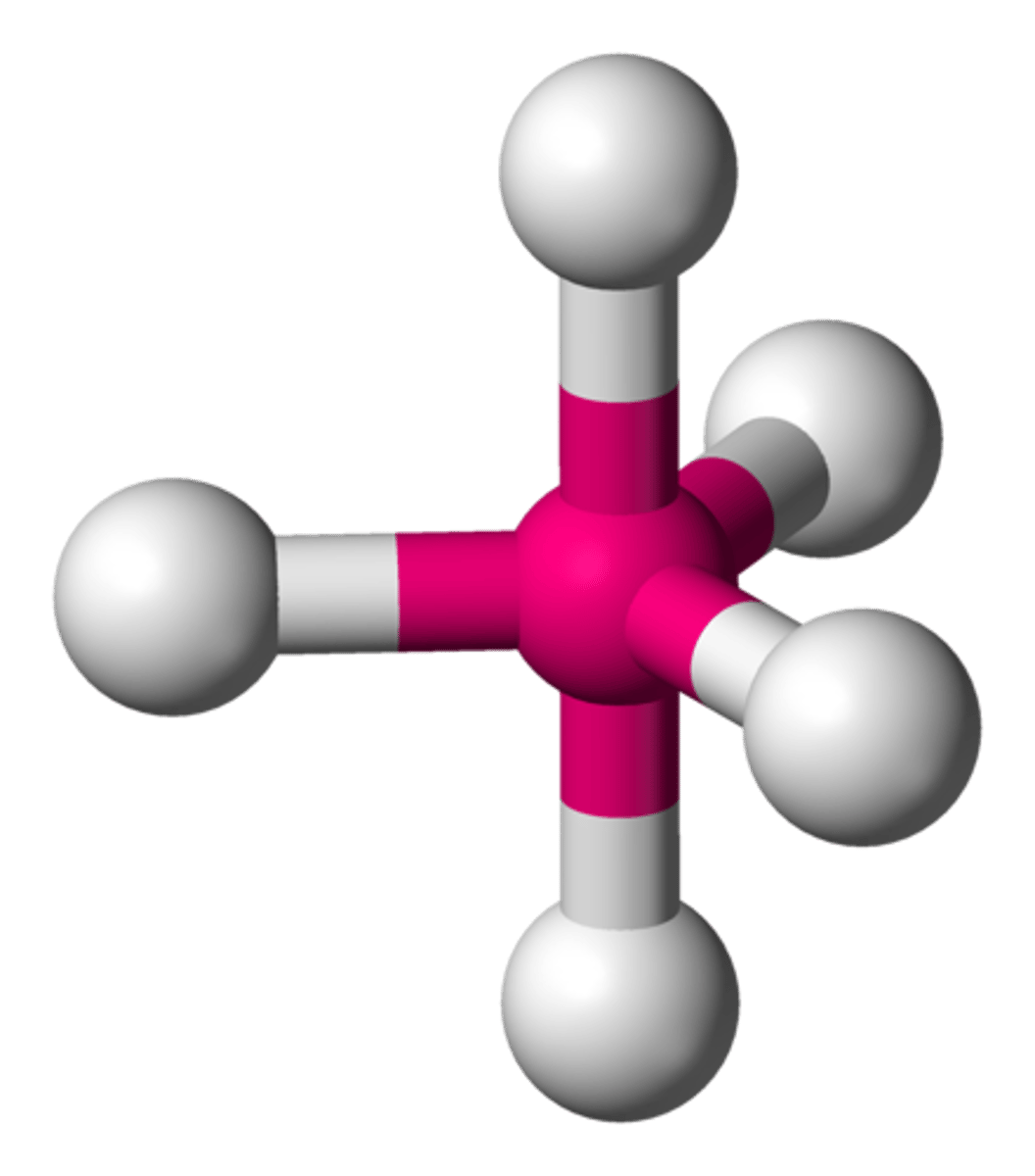
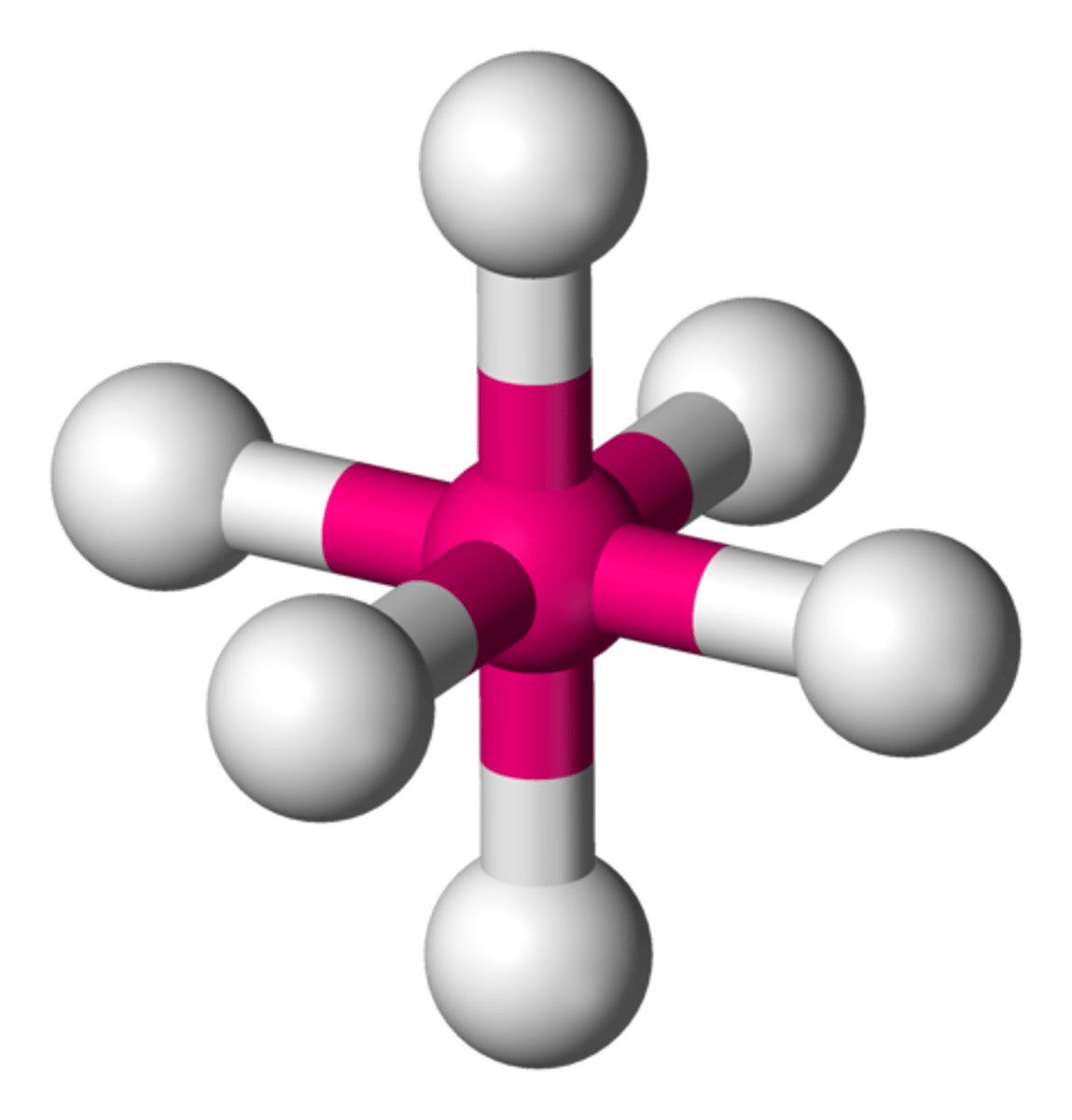
6 groups around central atom
octahedral (90)
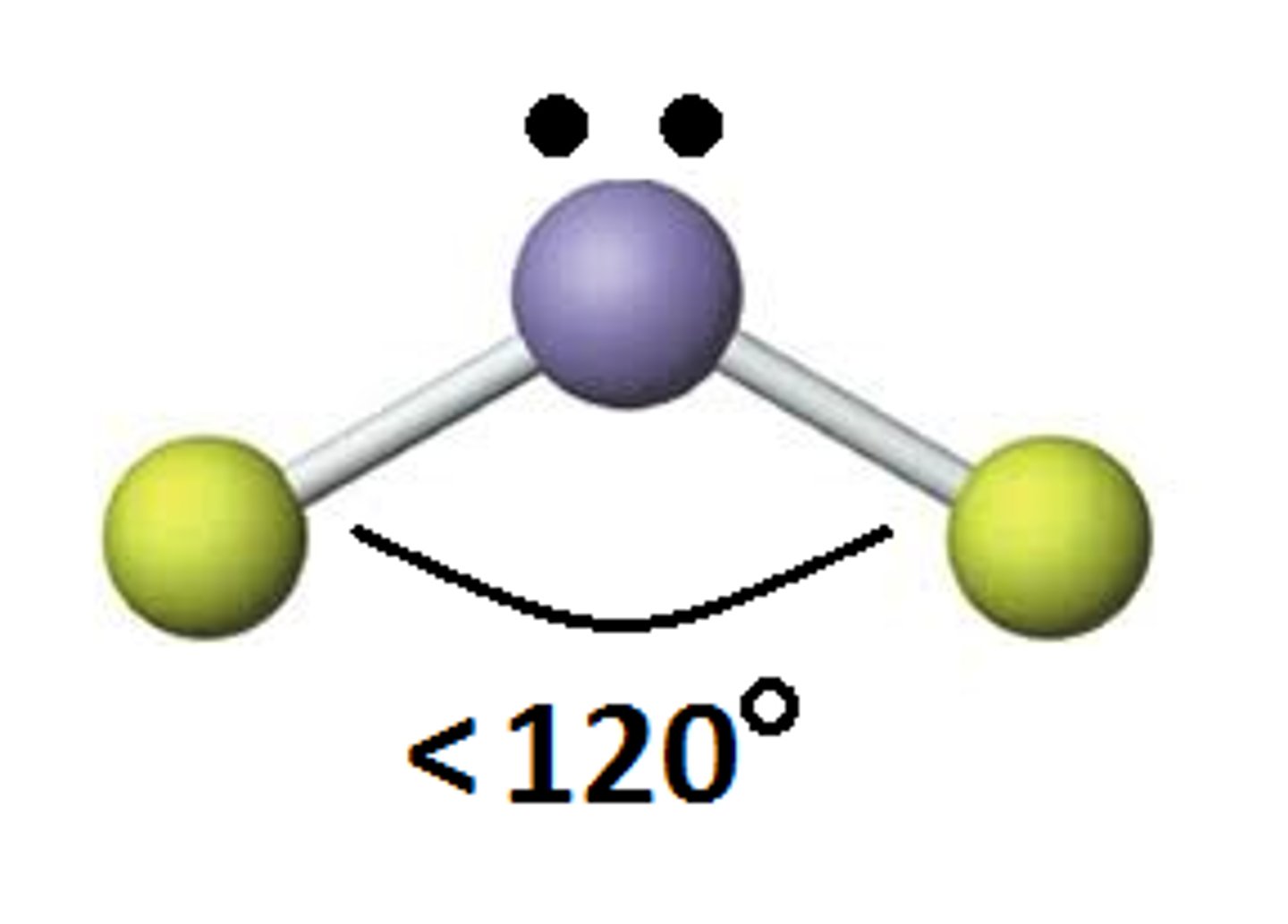
3 electron groups- 1 is lone pair
Trigonal planar, Bent (less than 120)
4 groups- 1 is a lone pair
tetrahedral, Trigonal Pyramidal (107)

4 groups- 2 groups are lone pairs
Tetrahedral, bent (105)
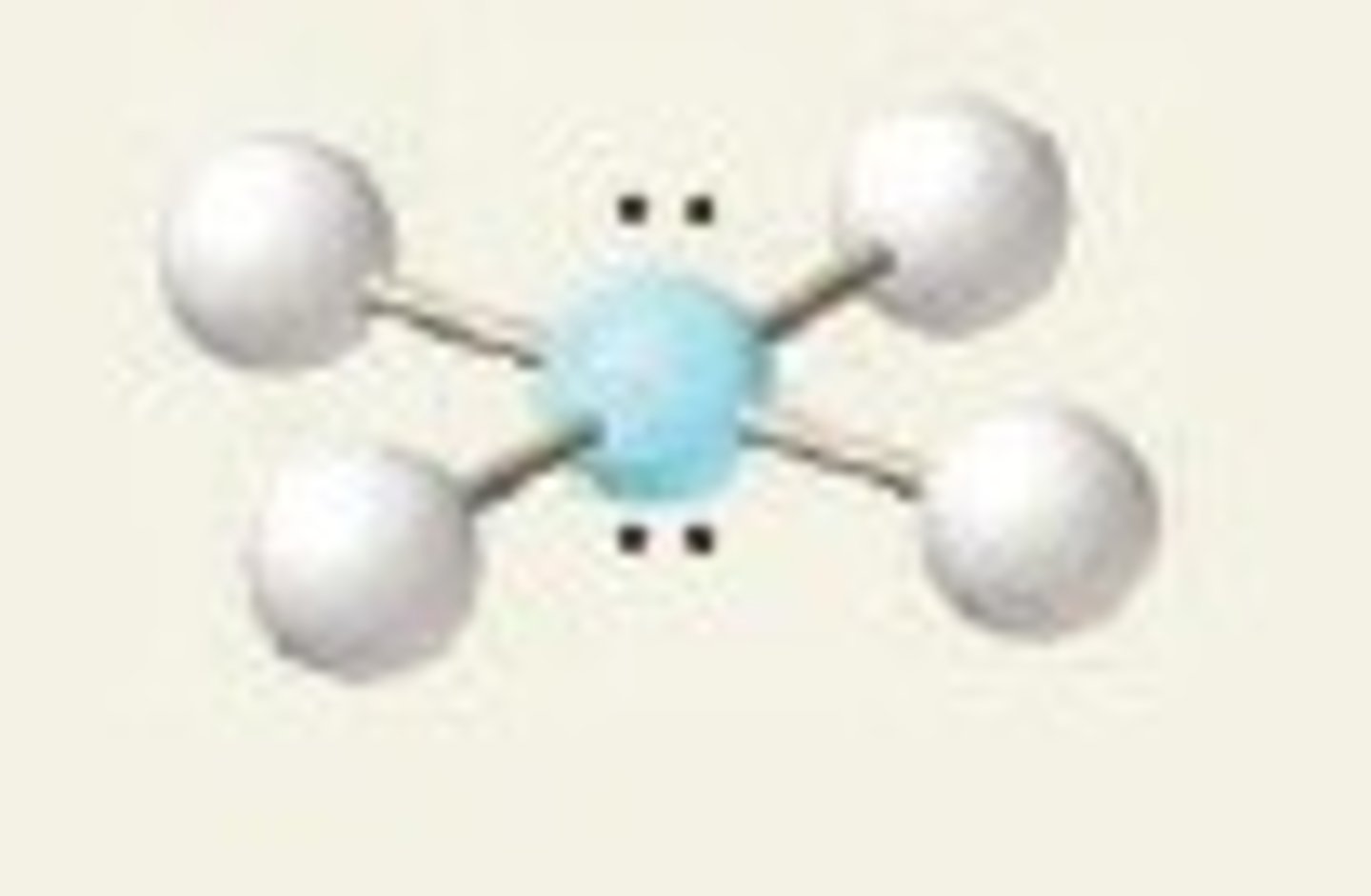
5 groups- 1 is a lone pair
Trigonal bipyramidal, Seesaw (101.5, 173.1)
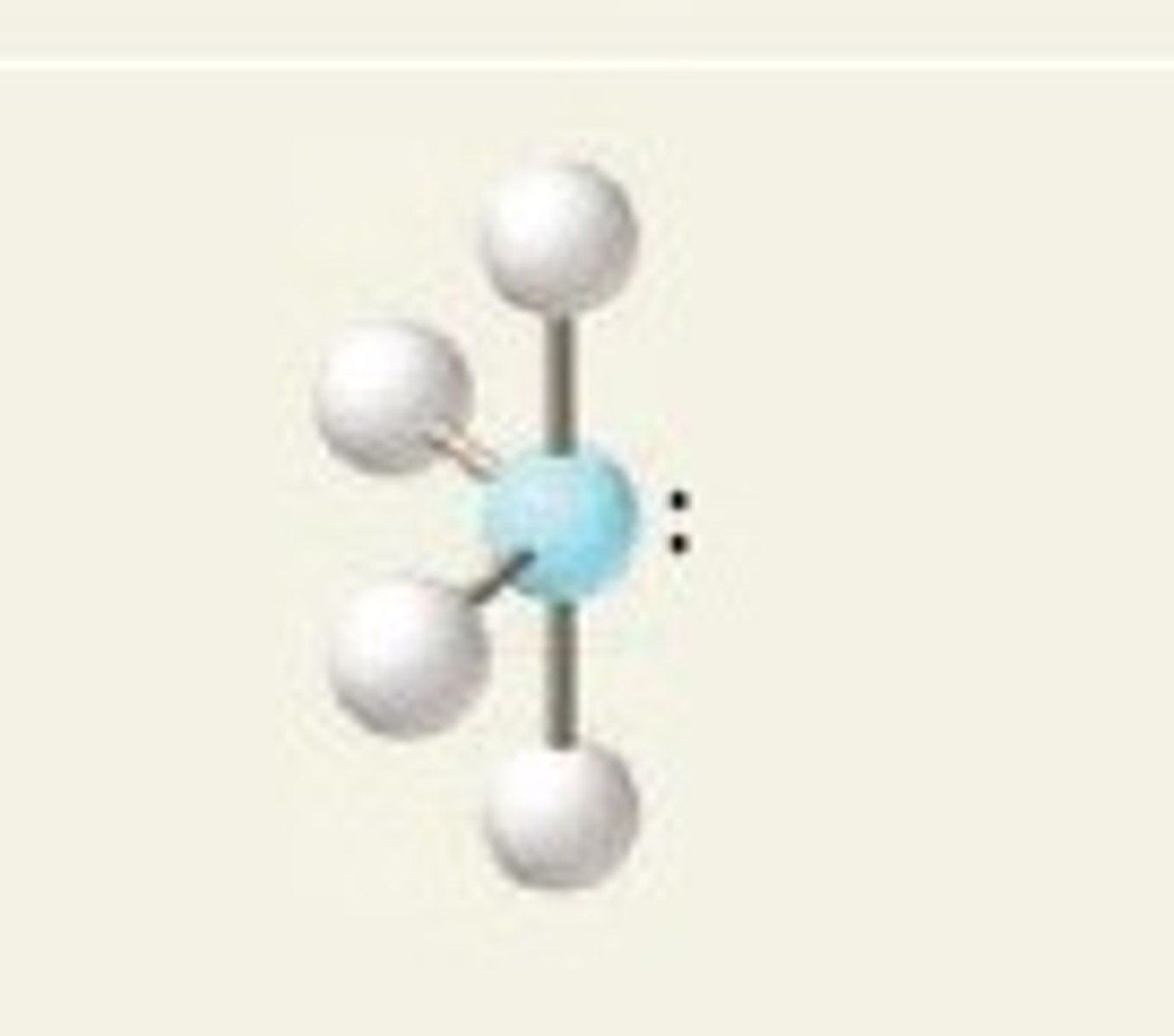
5 groups- 2 groups are lone pairs
Trigonal bipyramidal, T-shaped
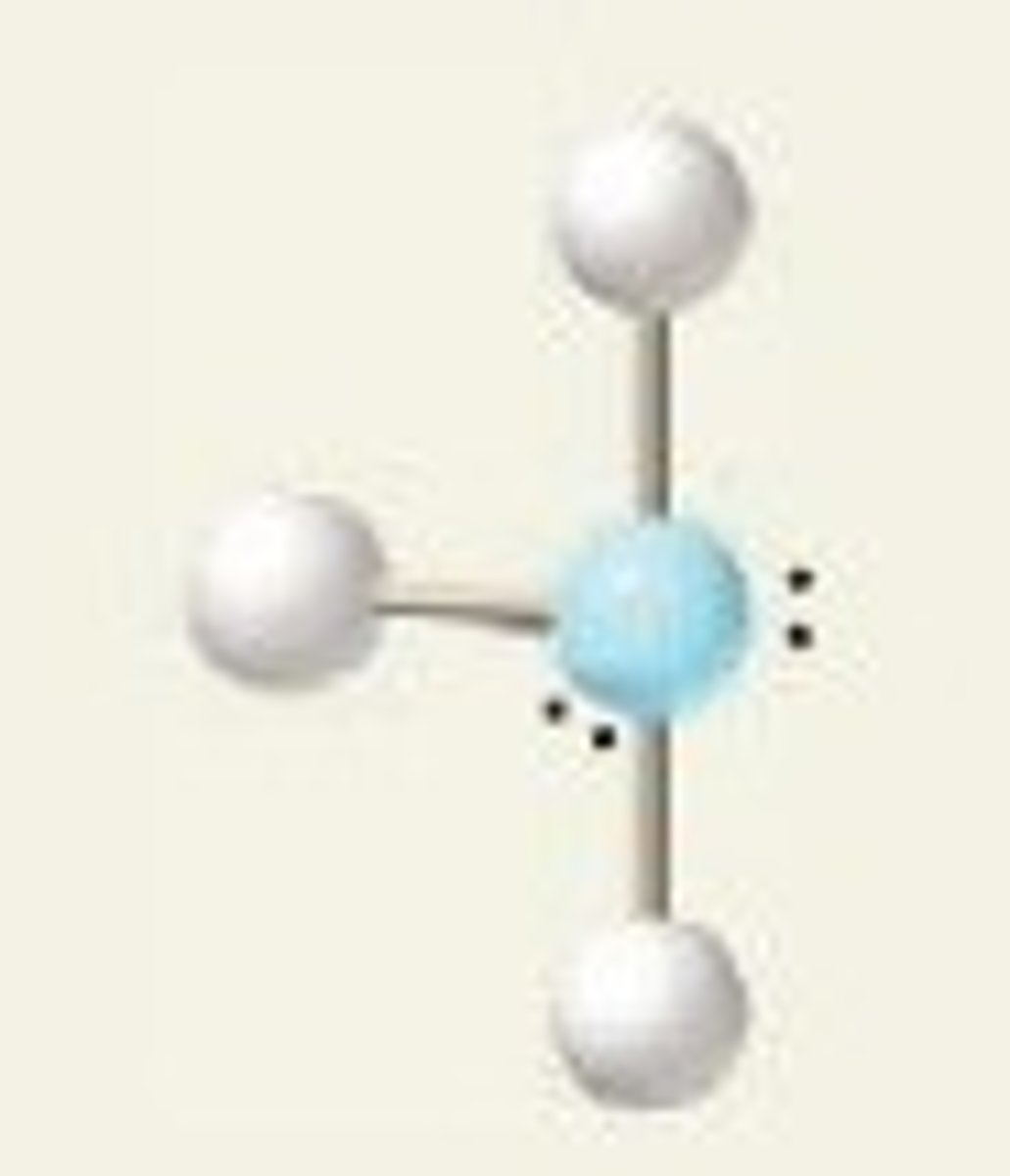
5 groups- 3 groups are lone pairs
Trigonal bipyramidal, Linear
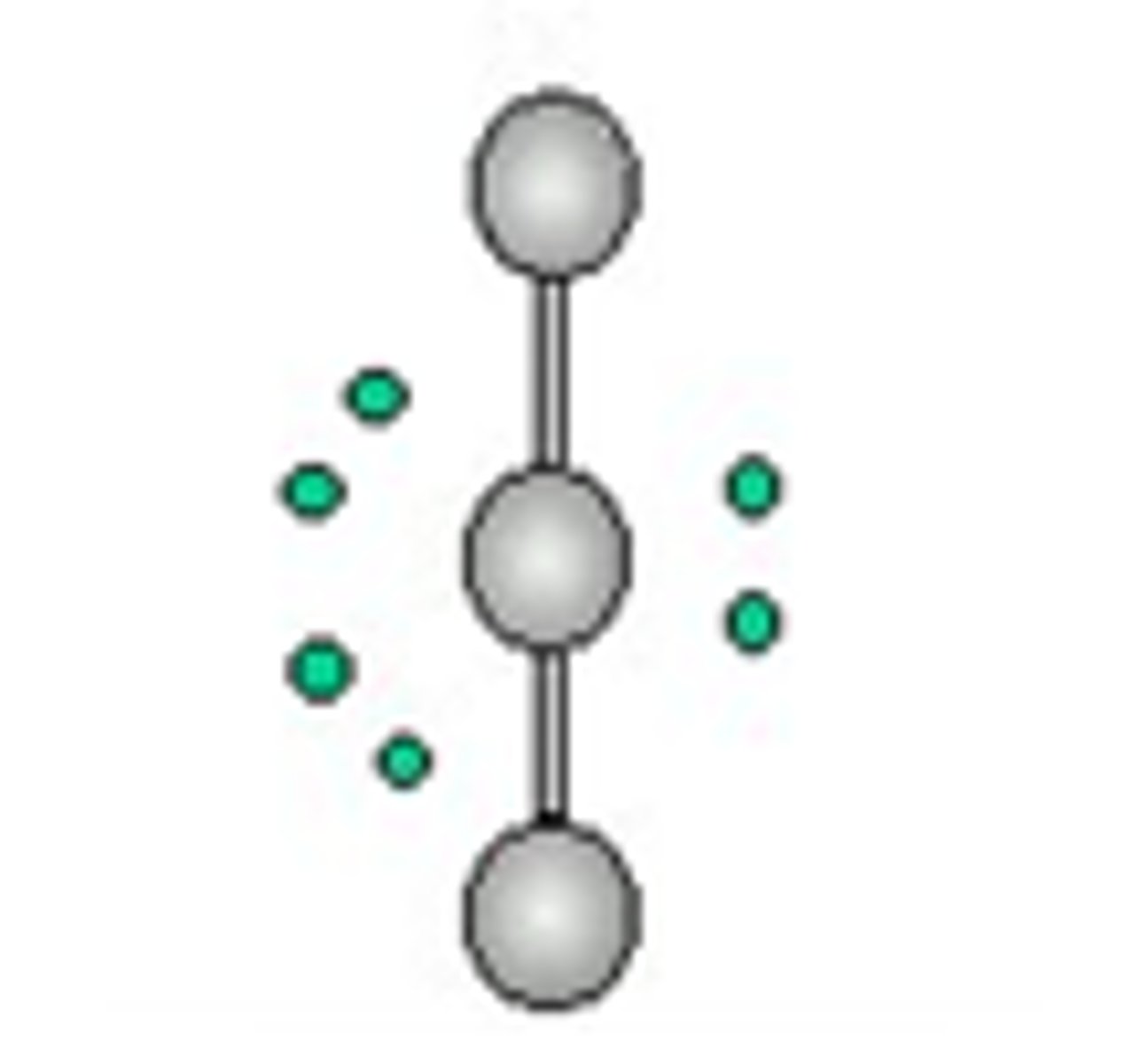
6 groups- 1 group is a lone pair
Octahedral, Square Pyramidal
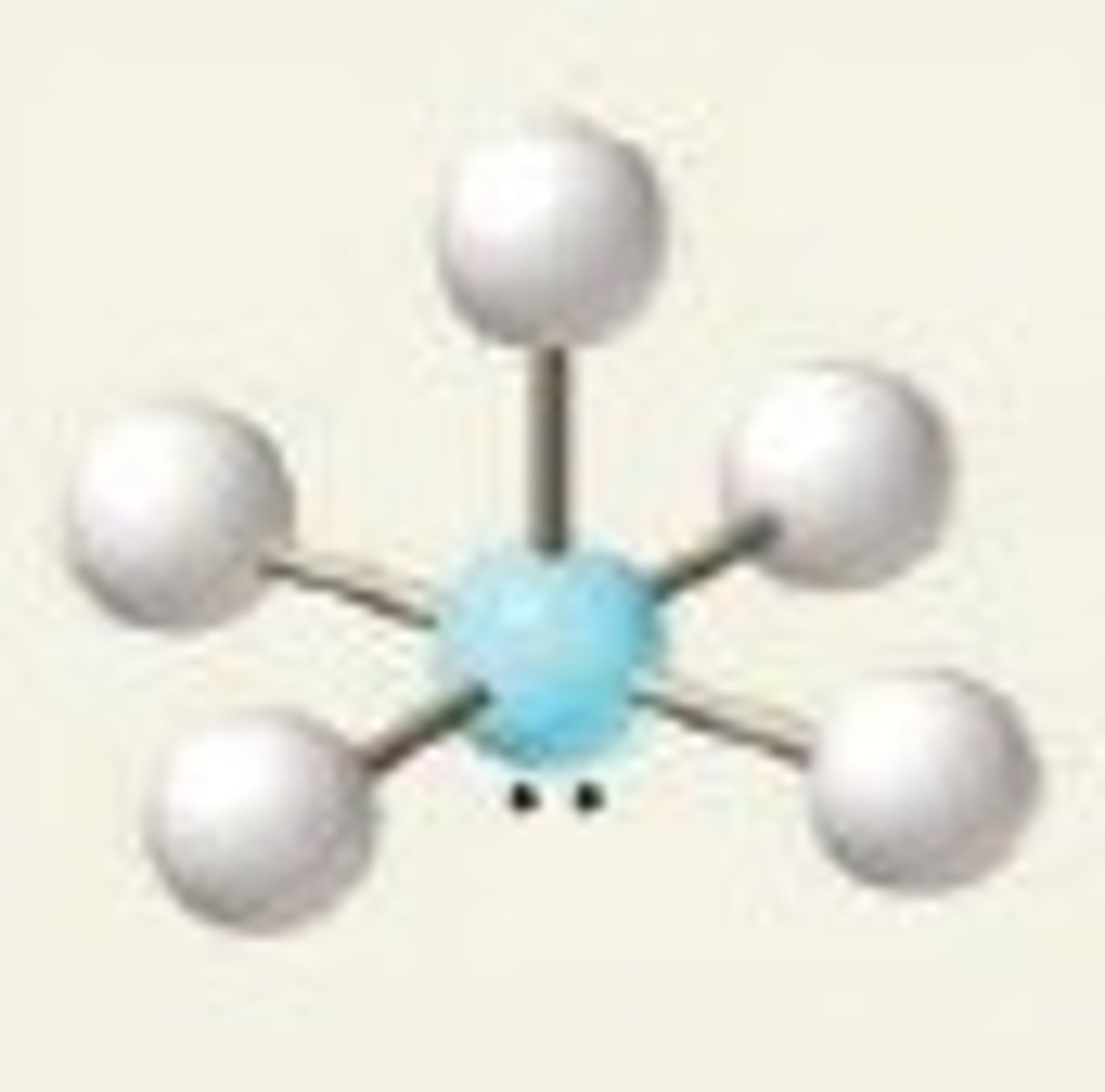
6 groups- 2 groups are lone pairs
Octahedral, Square planar
Linear
180
Trigonal Planar
120
Tetrahedral
109.5
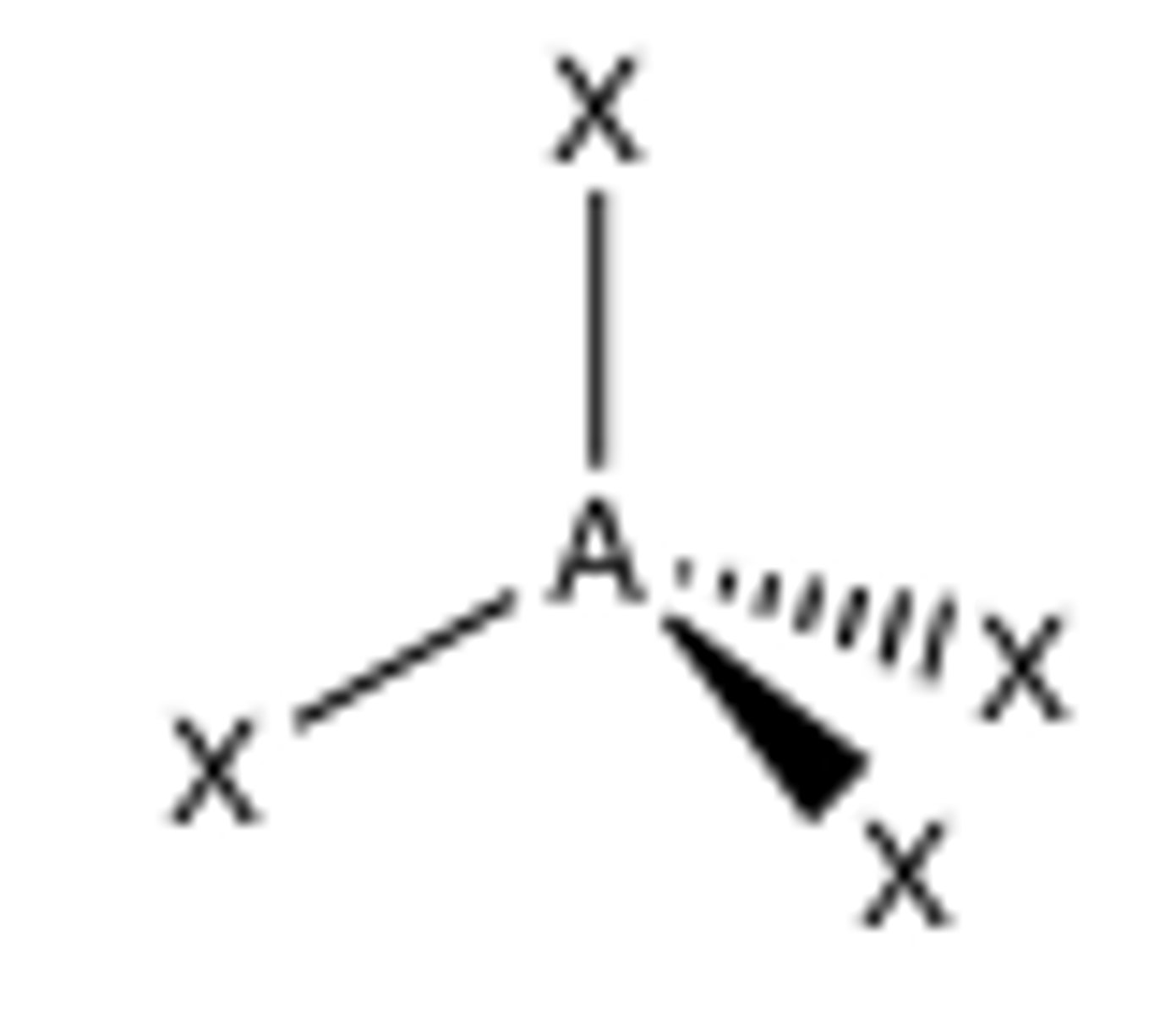
Trigonal Bipyramidal
90-120
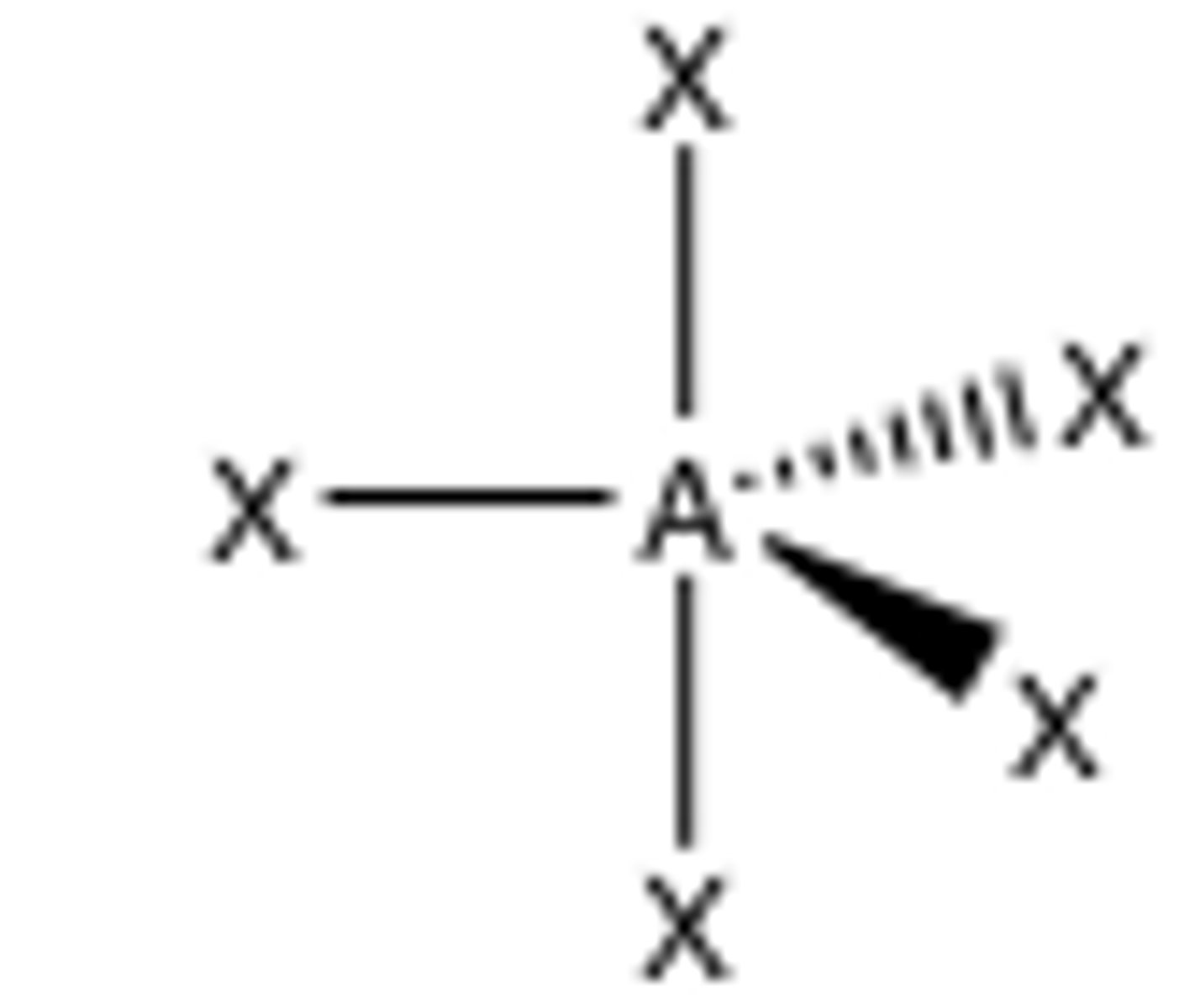
Octahedral
90
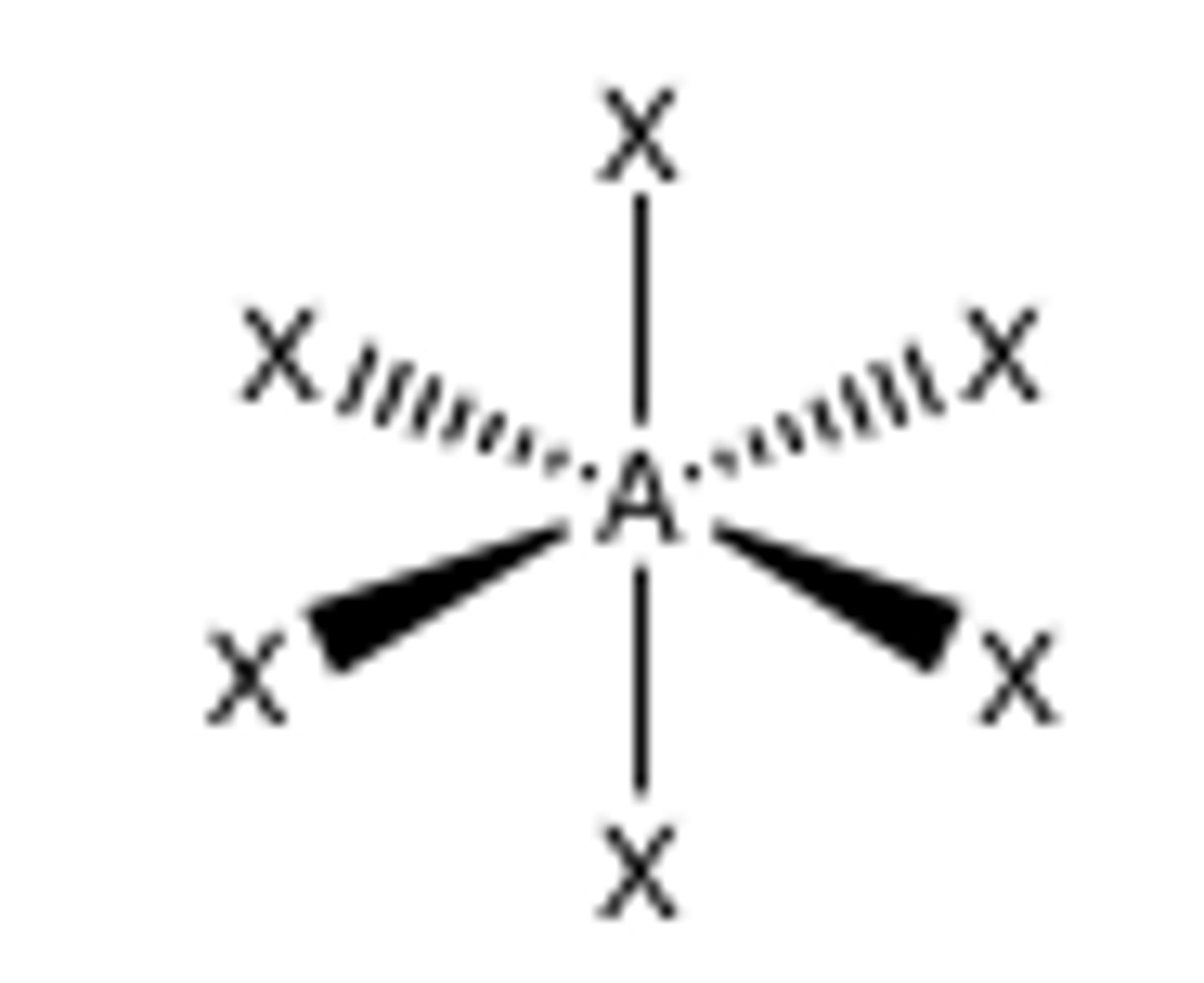
What does this deck go in the order of?
Electron, then molecular. Electron is basic, doesn’t take LP into account. Molecular does.