Chemistry 101
1/174
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
175 Terms
H
hydrogen
C
carbon
N
nitrogen
O
oxygen
F
flourine
Cl
chlorine
S
sulfur
P
phosphorous
Na
sodium
Mg
magnesium
K
potassium
Ca
calcium
Fe
iron
Cu
copper
Zn
zinc
Pb
lead
Li
lithium
Be
beryllium
B
boron
Ne
neon
Al
aluminum
Si
silicon
Ar
argon
Sc
scandium
Ti
titanium
V
vanadium
Cr
chromium
Mn
manganese
Co
cobalt
Ni
nickel
Ga
gallium
Ge
germanium
As
arsenic
Se
selenium
Br
bromine
Kr
krypton
Rb
rubidium
Sr
strontium
Ag
silver
Cd
cadmium
Sn
Tin
I
Iodine
Cs
cesium
Ba
barium
Pt
platinum
Au
gold
Hg
mercury
Xe
xenon
U
uranium
chemistry is the science that seeks to understand what matter does by studying what ___ and ____ do
atoms, molecules
hypothesis
a tentative, untested explanation of observations, experiments, or laws
model
a method of visualizing objects
theory
a refined and repeatedly verified hypothesis that is generally accepted. In science a theory is truth.
law
a summary of important relationships or information obtained from observations and experiments
a law provides the
what
a theory gives the
how
matter is defined as anything that occupies ___ and has ____
space, mass
definite
it stays the same no matter the container it is in
indefinite
it changes depending on the container it is in
pure substances are made of only ___ type of atom or molecule
one
mixtures are composed of __ or more different types of atoms or molecule combined in various compositions
two
elements
pure substances that consist of one kind of atom
There are currently __ known elements, of which _ are naturally occurring
118,89
solids are usually ___ on the periodic table
black
liquids are usually __ on the periodic table
blue
gases are usually ___ or ___ on the periodic table
red, green
atom
the smallest particle of an element
molecule
a group of atoms “stuck” together that act as a unit
H is a ___ molecule
diatomic
the diatomic molecules are
H, N, O, F, Cl, Br, and I
N is a ___ molecule
diatomic
O is a ___ molecule
diatomic
F is a ___ molecule
diatomic
Cl is a ___ molecule
diatomic
H is a ___ molecule
diatomic
Br is a ___ molecule
diatomic
I is a ___ molecule
diatomic
P has a subscript of
4
S has a subscript of
8
compounds are
made up of 2 more elements in a fixed proportion by mass
molecule
two or more atoms attached to each other that act as a unit
Homogeneous Mixtures
they are visually uniform, also called solutions
Heterogeneous Mixtures
they are not visually uniform
Identify the following as a/an
A.Element, B.Compound, C.Homogeneous Mixture, D.Heterogenous Mixture
S subscript of 8
element
Identify the following as a/an
A.Element, B.Compound, C.Homogeneous Mixture, D.Heterogenous Mixture
Pure sugar
compound
Identify the following as a/an
A.Element, B.Compound, C.Homogeneous Mixture, D.Heterogenous Mixture
C subscript 3 H subscript 8 O
compound
Identify the following as a/an
A.Element, B.Compound, C.Homogeneous Mixture, D.Heterogenous Mixture
Salt dissolved in water
homogeneous mixture
Identify the following as a/an
A.Element, B.Compound, C.Homogeneous Mixture, D.Heterogenous Mixture
Pure water
compound
Identify the following as a/an
A.Element, B.Compound, C.Homogeneous Mixture, D.Heterogenous Mixture
Tap water
homogeneous mixture
Identify the following as a/an
A.Element, B.Compound, C.Homogeneous Mixture, D.Heterogenous Mixture
a pineapple
heterogeneous mixture
Identify the following as a/an
A.Element, B.Compound, C.Homogeneous Mixture, D.Heterogenous Mixture
Pure copper
element
metals are usually shiny ,ductile (they can be shaped into ___), malleable, and good _____ of heat and electricity
wires, conductors
nonmetals are not shiny, ductile, nor malleable, and they are often ___ conductors of heat and electricity
poor
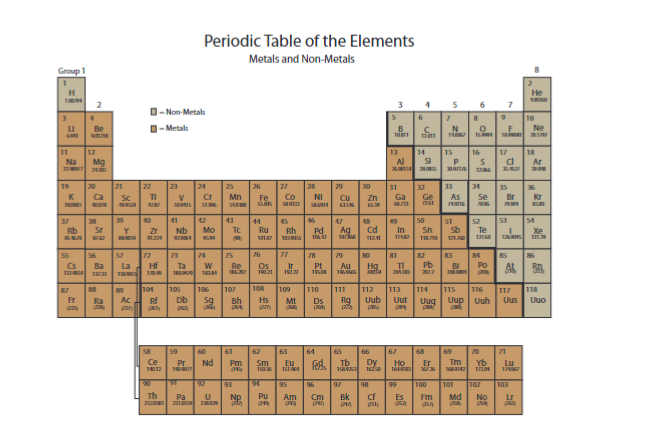
Are the metals on the left or the right? What about nonmetals?
left, right
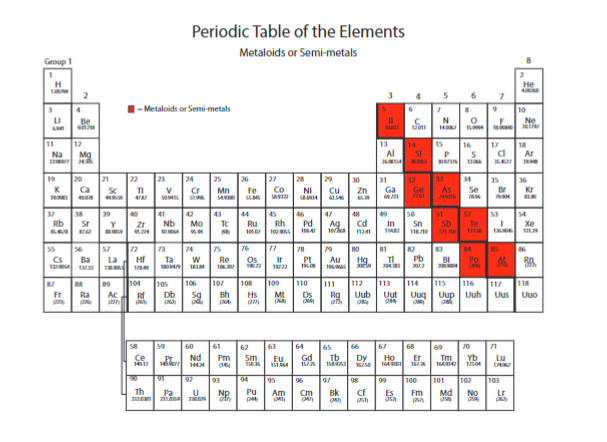
What are the highlighted called?
metalloids or semimetals
Name the Metalloids or semimetals
B, Si, Ge, As, Sb, Te, Po, At
groups or families are elements in the same ___. These elements have similar chemical and physical properties.
column
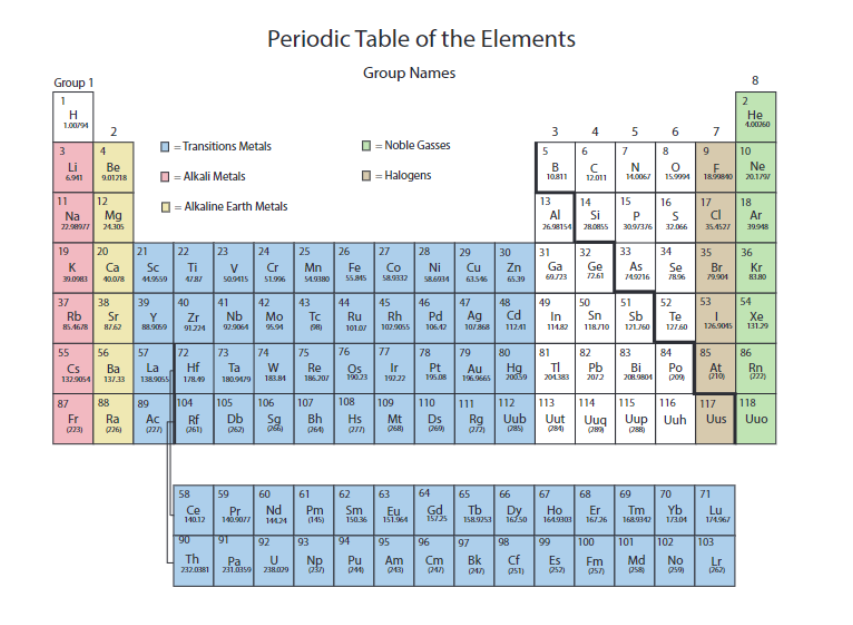
The alkali metals are in
pink
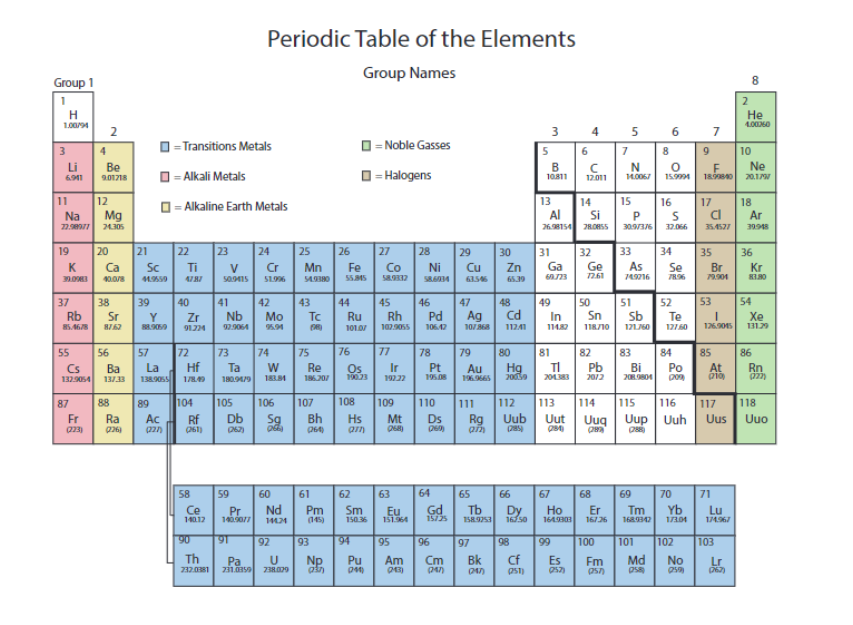
the alkaline earth metals are in
yellow
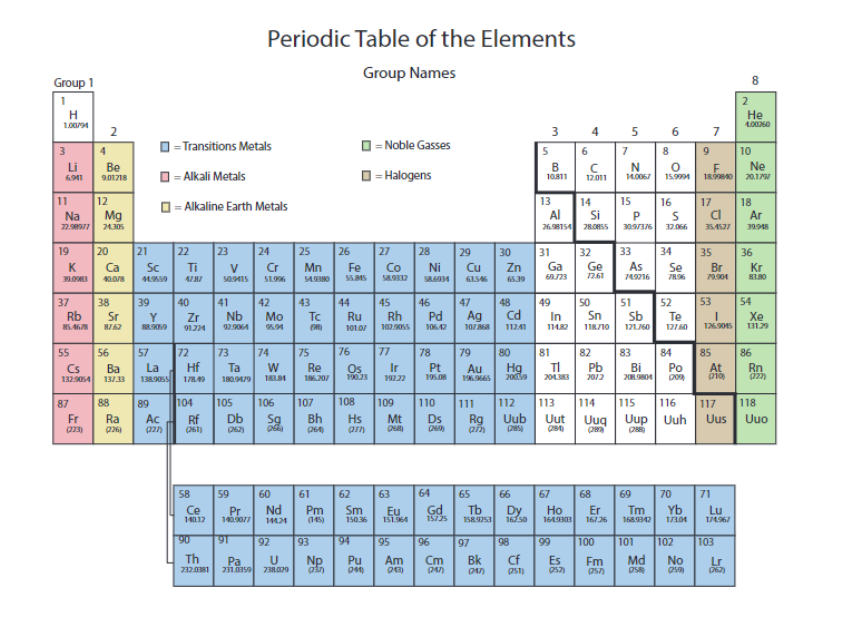
the transition metals are in
blue