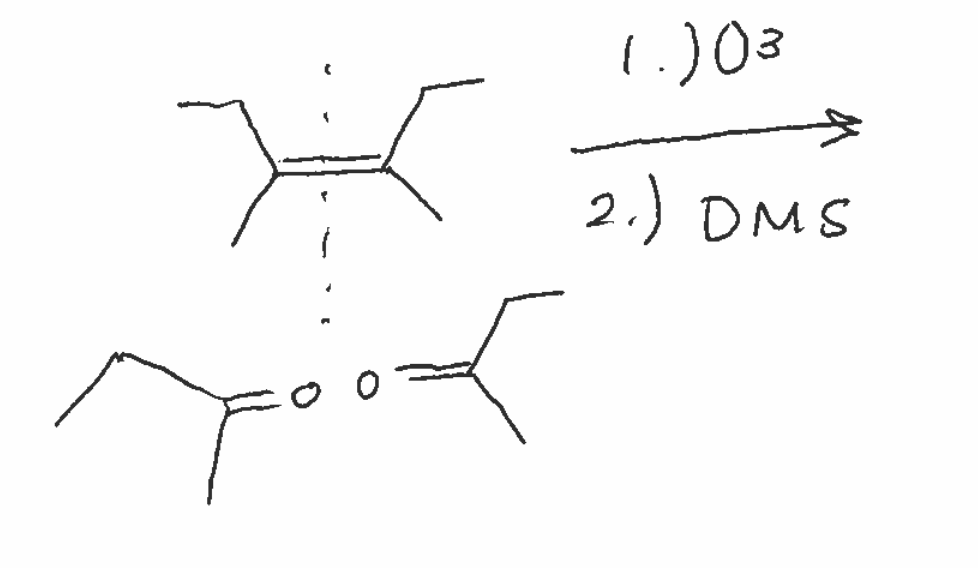alkene reactions
1/16
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
17 Terms
hydrohalogenation reagents
Hydrogen and a halogen -
HBr, HCl, etc (markovnikov)
HBr/ROOR (anti-markovnikov)
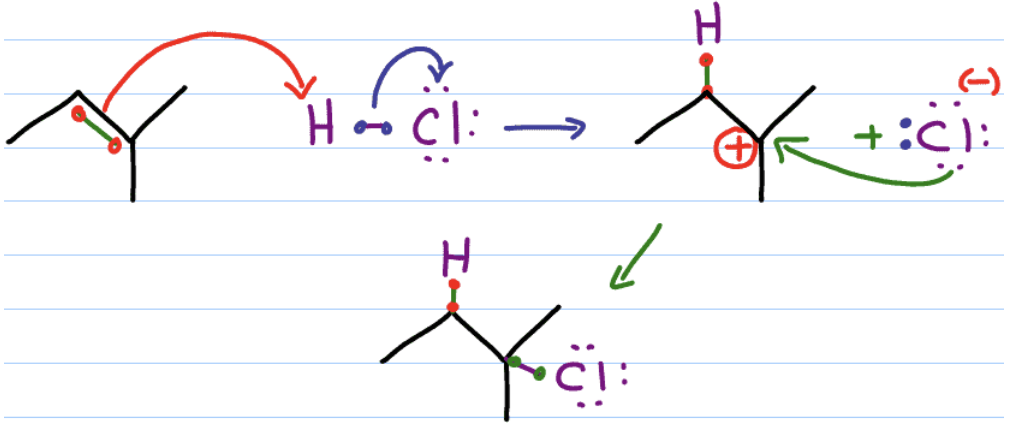
Hydrohalogenation mechanism
regioselectivity
Hydration reagents
H3O+, [H+]/H2O, [H2SO4] (markovnikov)
1)BH3 THF/H2O2, NaOH (anti markovnikov)
Hydration - acid catalyzed reaction
H+]/H2O, [H2SO4]
markovnikov position
can be rearrangements
![<ul><li><p>H+]/H2O, [H2SO4]</p></li><li><p>markovnikov position</p></li><li><p>can be rearrangements</p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/3ca9c40e-b667-4b5c-834d-5ed38d0ee0ee.png)
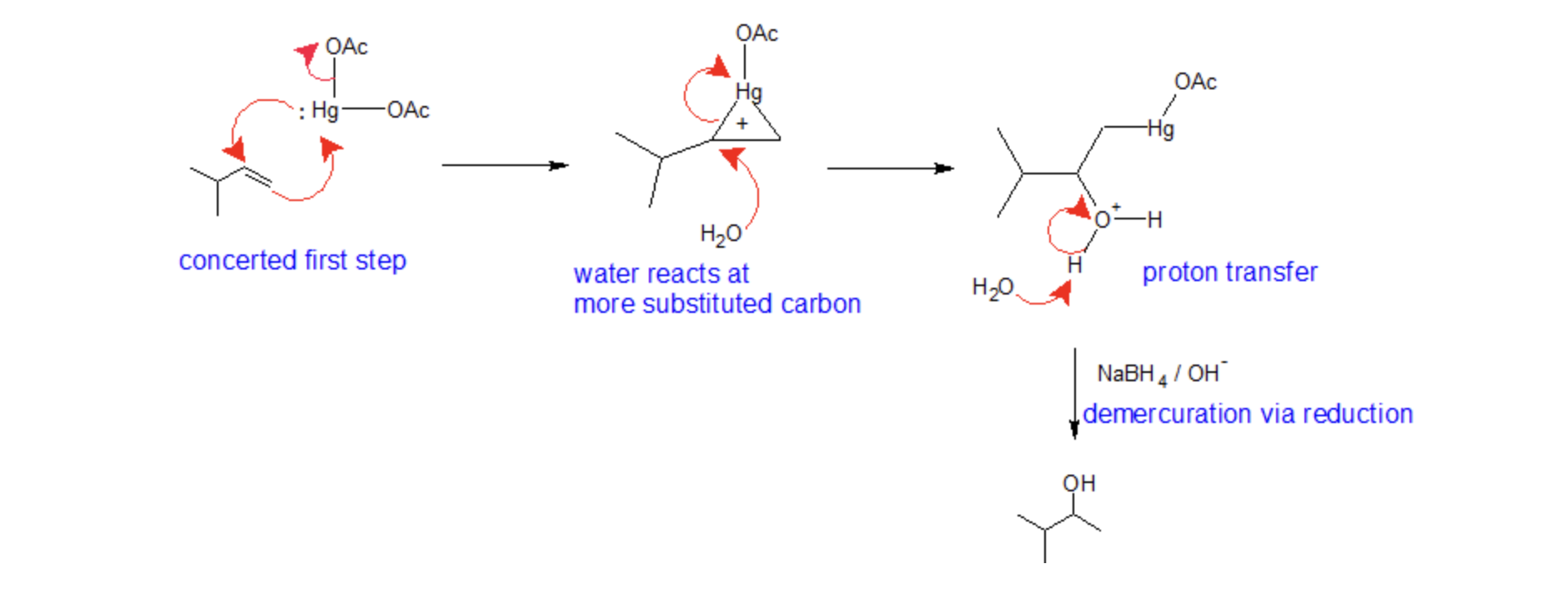
Hydration - oxymercuration/demercuration reaction
1) 𝐻𝑔(𝑂𝐴𝑐)2, THF, H2O/ NaBH4
markovnikov position
no rearrangements
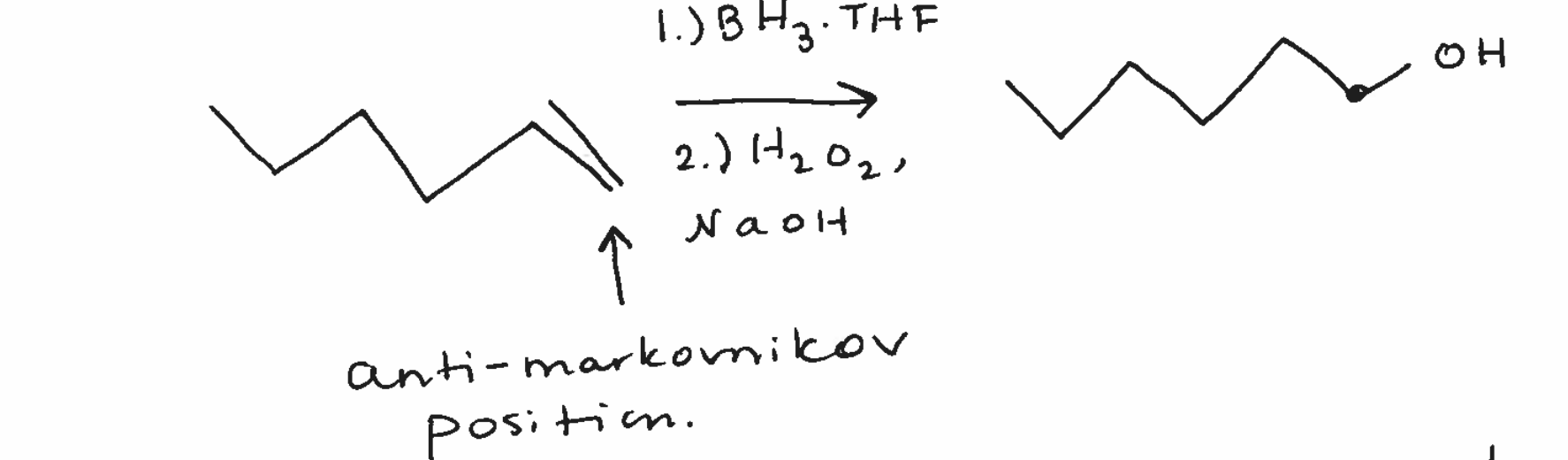
Hydration - hydroboration/oxidation
forms double bond on anti-markovnikov position
syn products if 2 chiral centers
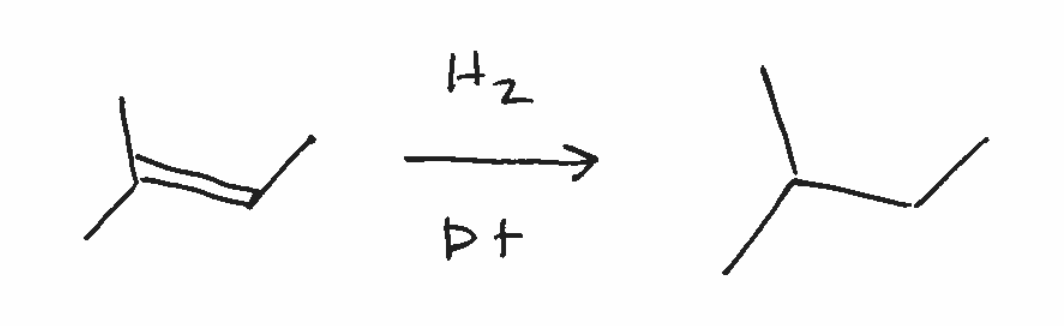
catalytic hydrogenation (dont need to know mechanism)
adds H/H across double bond
syn products if 2 chiral centers
Halogenation
forms bridge intermediate
forms anti products with chiral centers
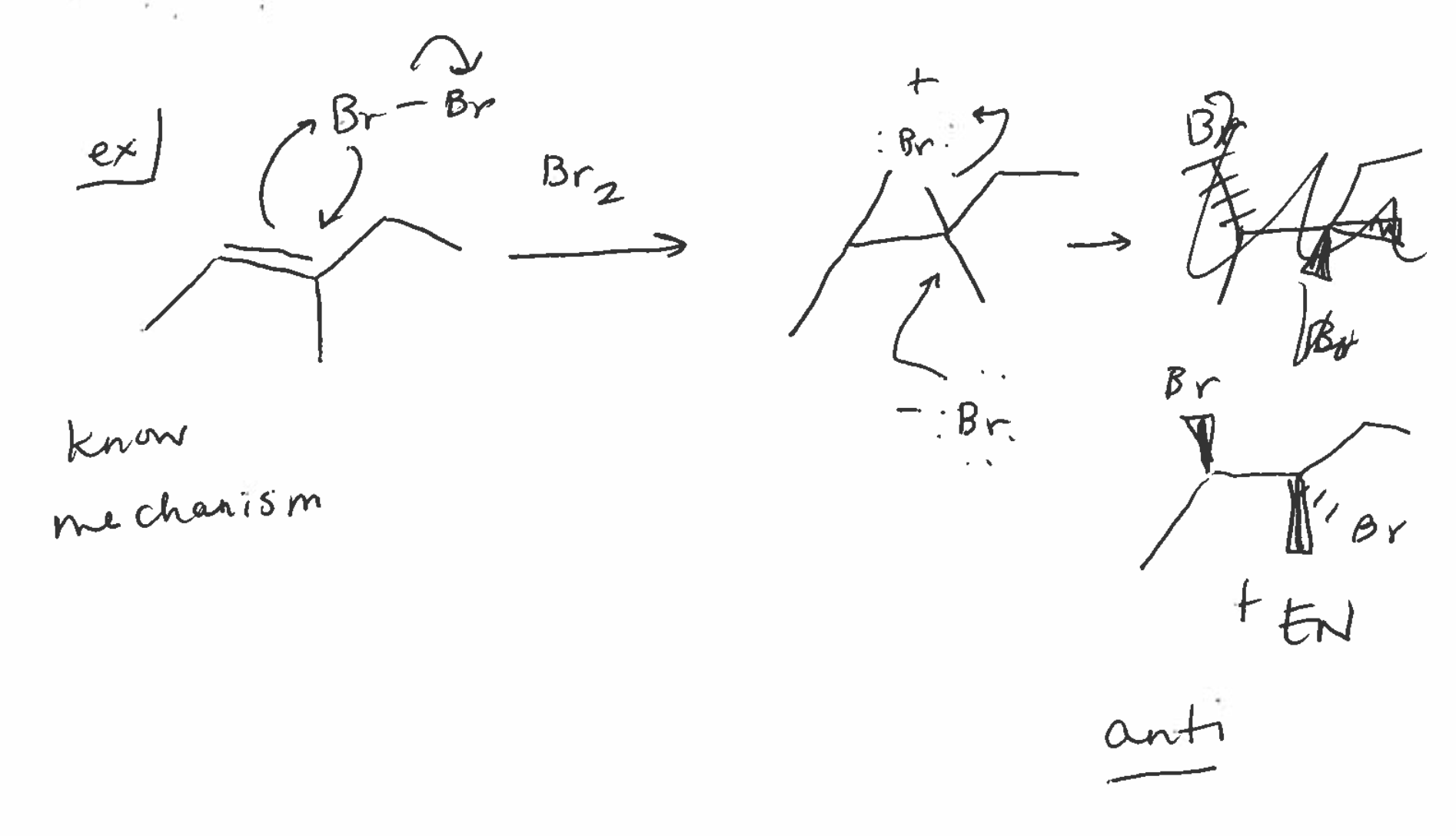
Halogenation reagents
di-halogens
ex) Br2
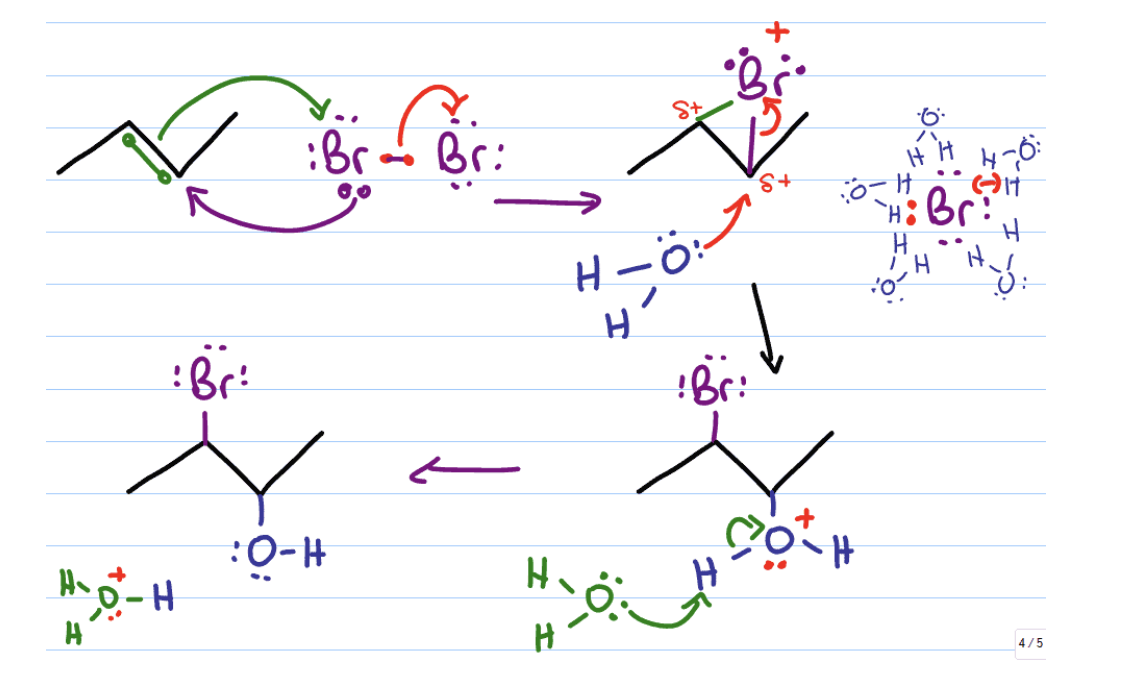
halohydrin formation reagents
di halogens + water
ex? Br2/H2O
halohydrin formation mechanism
OH group on more substituted carbon
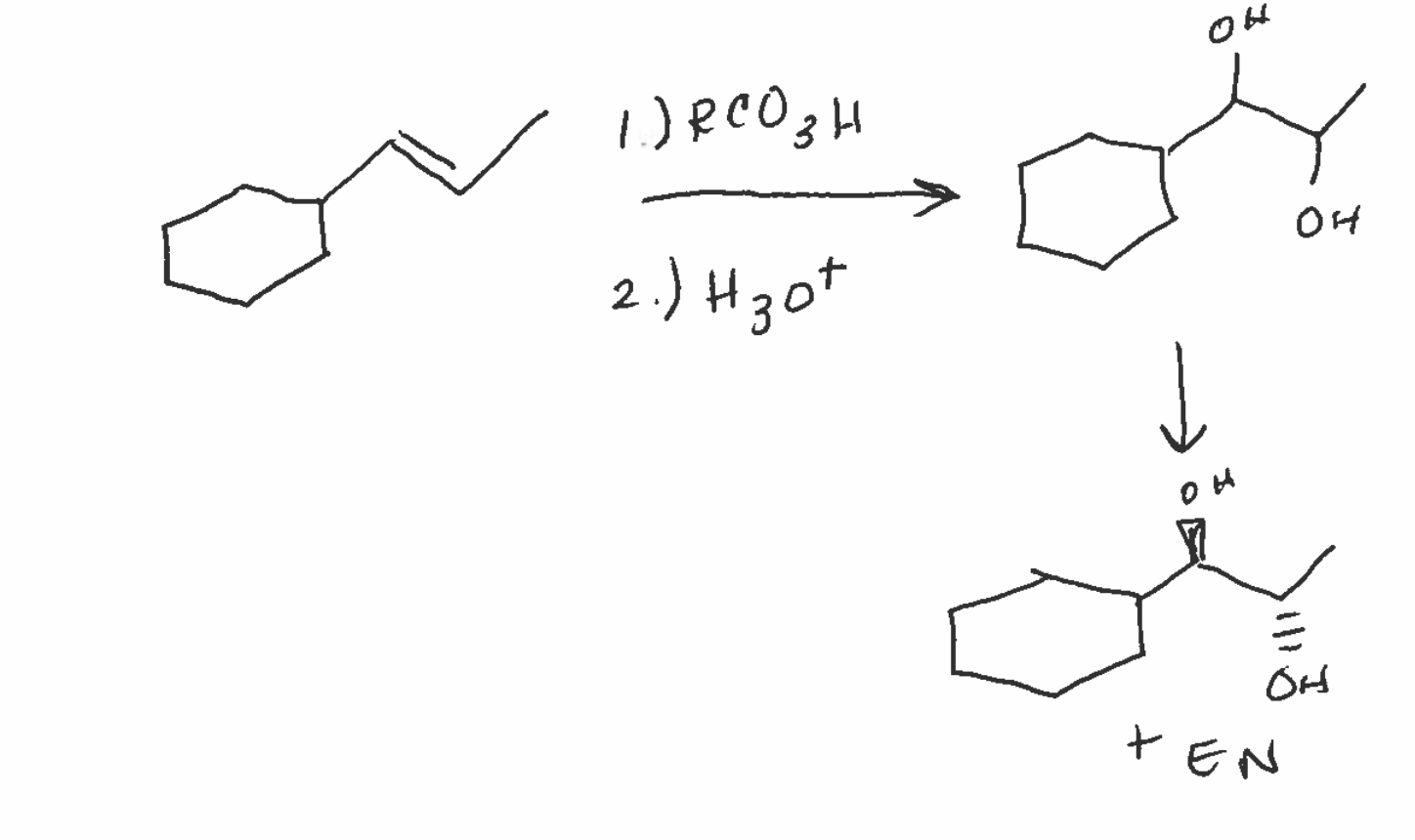
Anti-dihydroxylation reagents
1) RCO3H / 2H3O+
anti-dihydroxylation (dont need to know mechanism)
anti products if chiral centers
syn-dihydroxylation reagents
OSO4 / NMO
OSO4 / NaHSO3, H2O
KMNO4, NaOH / cold
syn-dihydroxylation (dont need to know mechanism)
forms syn products
Oxidative cleavage reagent
O3 / DMS
Oxidative cleavage mechanism