Biomaterials MAP2
0.0(0)
Card Sorting
1/197
There's no tags or description
Looks like no tags are added yet.
Last updated 7:37 PM on 6/15/23
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
198 Terms
1
New cards
What are the sequences of events following the implantation of medical devices?
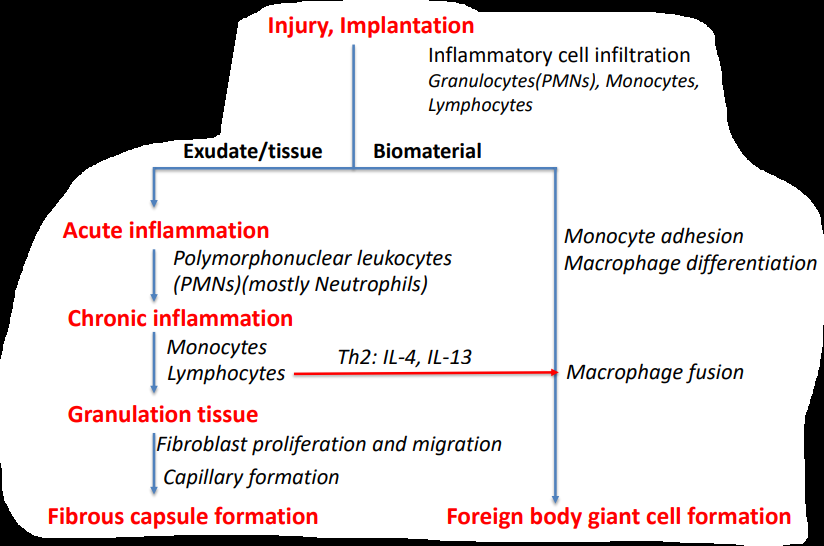
2
New cards
How is acute and chronic inflammation compared?
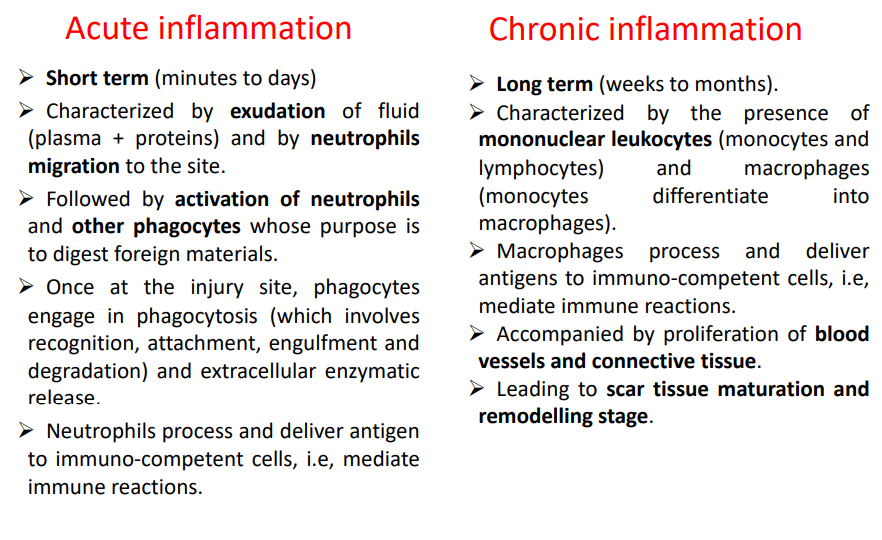
3
New cards
T and B cells
lymphocites, the former takes part of the cell-mediated immune response, the latter takes part in the humoral immune response (antibody production).
4
New cards
Natural killer cells (NK cells)
cytotoxic lymphocyte that constitutes a major component of the innate immune system, kills by releasing proteins that cause the target cell to die by apoptosis.
5
New cards
Deine chemokines and chemotaxis
chemotactic cytokines directed cell locomotion
6
New cards
Define cytokines
Cytokines - small cell-signaling proteins
7
New cards
What is the antigen-antiboy complement? What are antigen-presenting cells?
Activation of complement by the antibody-antigen bound pair via linkage to a molecule in the complement, resulting in the formation of complement fragments, which are potent chemotactic agents for polymorphonuclear leukocytes or facilitate phagocytic cell recognition. Dendritic cells find and capture antigens and present them to lymphocytes.
8
New cards
How are pathogens that are inside cells, such as virus , reached by the immune system?
n T-lymphocytes (T cells) operates. T cell surface receptors recognize an antigen that is coupled to a molecule on the cell surface. This binding leads to phagocytosis. These cell surface molecules are part of the major histocompatibility complex (MHC). T-helper cells can recognize antigen-MHC molecules binding and release powerful cytokines, including g interferon, which assist in macrophage activity.
9
New cards
What are antibodies?
Antibodies (also known as immunoglobulins, Ig) are made and carried out by B-lymphocytes (B cells). These are programmed to make a single type of antibody . Antigen: part of the microorganism that evokes an antibody response, via a specific binding. Lymphocytes with antigens bound at their surface receptor, develop into an antibody-forming plasma cell, which then secretes identical antibodies. The lymphocytes undergo successive waves of proliferation.
10
New cards
How is inflammation mediated?
Release of pro-inflammatory mediators induces changes in the endothelial cells of the microvasculature, increasing the permeability of the vessel walls. There will be an influx of leukocytes and plasma. Neutrophils arrive very early, followed by monocytes. Neutrophils release protease-laden granules and ROS, should the cause of injury be a bacteria or non-microbial (e.g. biomaterial-derived particles). In the second case ECM and resident cells may be destroyed. After this release, the neutrophils die by apoptosis and are cleared by macrophages that arrive a little later to the inflamed site
11
New cards
What are pattern recognition receptors?
Pattern-recognition receptors (PRR) are expressed by cells of the innate immune system, including: macrophages, monocytes, dendritic cells, neutrophils and epithelial cells. They primarily: i) Probe extracellular space and endosomal compartments for pathogen-associated molecular patterns. Signal transduction from these receptors promotes the production of pro-inflammatory cytokines and chemokines. ii) Recognize microbial and non-microbial molecular patterns, activating inflammasomes The net result of the processes (molecule recognition, signal transduction and gene expression) is the activation of resident macrophages and other cells and release of cytokines and other mediators of inflammation. This culminates in the resolution of inflammation or infection. Perpetuation of inflammasome activation can lead to chronic inflammatory diseases.
12
New cards
How are inflammosomes formed?
Inflammasome formation is triggered by a range of substances that emerge during infections, tissue damage or metabolic imbalances. Once the protein complexes have formed, the inflammasomes activate caspase 1, which proteolytically activates the proinflammatory cytokines interleukin-1β (IL-1β)3 and IL-18.
13
New cards
What are inflammosomes?
The inflammasomes are innate immune system receptors and sensors that regulate the activation of caspase-1 and induce inflammation in response to infectious microbes and molecules derived from host proteins
14
New cards
What are the principal components of innate immunity?
Innate immunity principal components: Anatomic barriers (skin and mucous membranes) Physiological barriers (temperature of body, low pH in stomach) white blood cells: Phagocytic cells (granulocytes (eosinophil, basophil, neutrophil))Natural killer cells Circulating proteins (such as complement and coagulation factors) Cytokines (small signaling proteins that regulate the overall cellular response)
15
New cards
What is cell response and humoral immunity?
Humoral Immunity: based on the action of antibodies (detection of foreign agents, such as bacteria) Cellular response: utilizes specialized lymphocytes (T cells) for the detection detection of altered self cells (from viral infections or cancer)
16
New cards
What are the two types of immune response?
Immune system components: Innate (or non-specific)_activatable/mobilizable immediately upon exposure to an invading agent. Is not-antigen-specific. Most the times associated to biomaterials. Adaptive/acquired (specific)_antigen-specific. It takes some time to respond but has immunological memory (faster response upon re-exposure). Relevant for biomaterials when these contain antigens (confined to biomaterials based on foreign proteins, including xenogenic and allogenic systems)
17
New cards
What are the 6 main mechanisms that contribute to the overall host response?
Physical/mechanical systems
Chemical systems Scale/size of the biomaterial (macroscopic, microscopic and nanoscopic)
harmacological systems
External systems (e.g. clinical factors, patient variability)
Chemical systems Scale/size of the biomaterial (macroscopic, microscopic and nanoscopic)
harmacological systems
External systems (e.g. clinical factors, patient variability)
18
New cards
What is the essence of biomechanical compativility mechanisms?
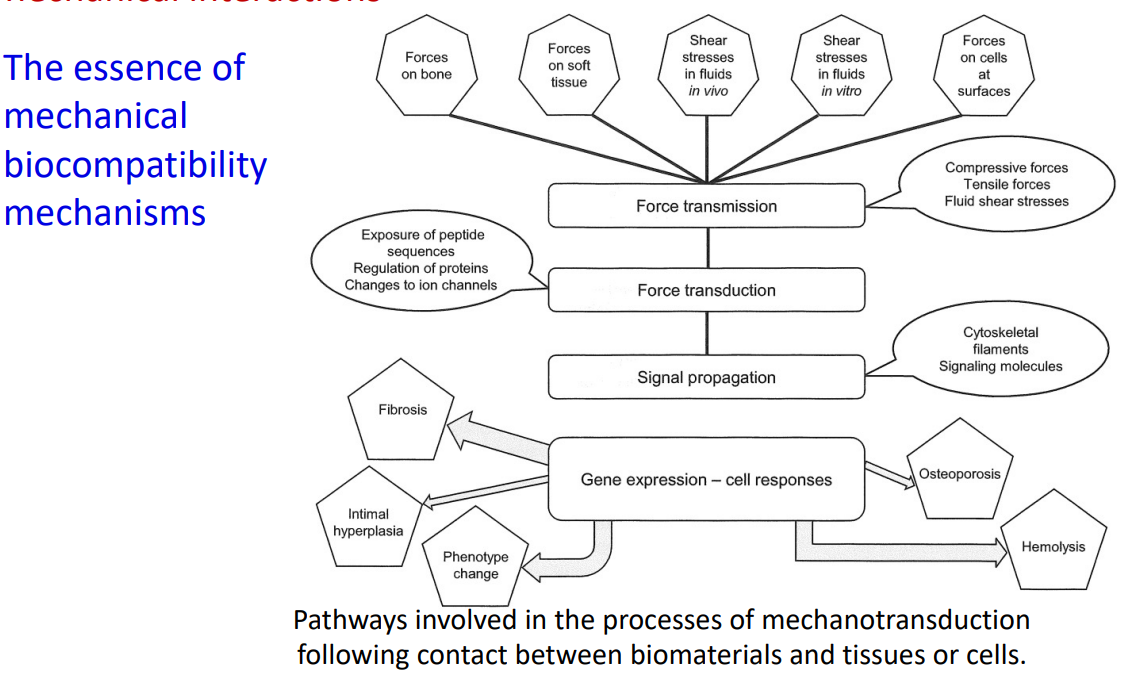
19
New cards
What are the 4 main question for assesing the incompatibility of a clinical case?
Is the host response determined by direct chemical interaction ? mechanical forces? Release of particulate or soluble substances ? Is the host response significantly influenced by presence of pharmaceutical agent? generation of new tissue ? microscale or nanoscale features ? Does the host response involve direct contact with the circulatory system ? Are there systemic or remote site components of the host response ?
20
New cards
What is the biomatiral implant pathway ?
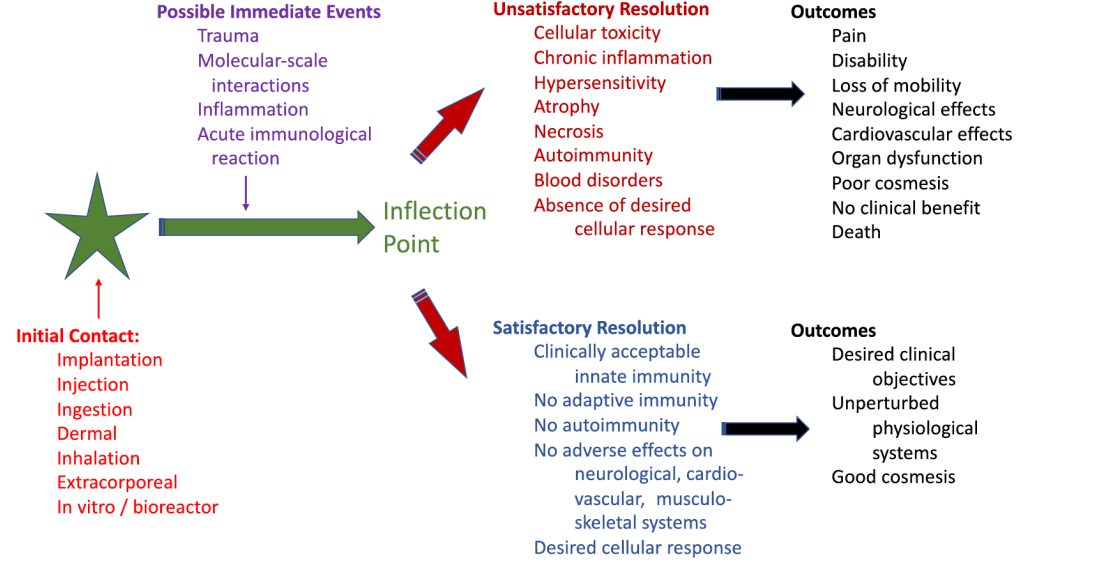
21
New cards
What is DLVO?
DLVO (Derjaguin, Landau, Verway, Overbeek) theory: a basic adhesion model developed for colloidal particles It does not take into account specific receptor-ligand interactions nor the surface topography. It may be used to model bacterial adhesion to biomedical implants.
22
New cards
How would you describe the ECM?
ECM can be described as a fiber reinforced matrix: i) fiber forming elements (collagen and elastin) ii) space-filling molecules (glycoproteins and proteoglycans) Fibronectin and laminin are two representative examples of glycoproteins that possess binding domains for cells iii) free and sequestered soluble mediators such as growth factors (stimulate a variety of cellular processes).
23
New cards
What cell contacts are important for inflammatory response?
Mucin-selectin and CAM-integrin, because they are important in cell migration that occurs early in the inflammatory response
24
New cards
What are selectins and mucins?
Selectins: cell-cell binding (heterophilic binding), binding to certain carbohydrate groups Mucins: ligands for selectins (glycosylated proteins: contain carbohydrate moieties)
25
New cards
What are the 5 cell contacts?
Tight juctions: cell-cell, not even small molecules can pass through Demosomes: cell-cell cadherins. They undergo calcium-dependent homophilic binding (binding between cadherin receptors of different cells) gap junctions: cell-cell, connecxins Hemidesmosome: cell-ecm, half an desmosome, integrins focal junction: integrins
26
New cards
Give two examples of protein coatings and its properties
Albumin: high water solubility and stability. Non adhesion to platelets and proteins. Heparin: immobilized locally and covalently. Inhibitor to platelet and thrombin adhesion.
27
New cards
What are three strategies for generating non-fouling surfaces?
i) Nonionic hydrophilic materials (e.g. PEG-based materials) ii) Zwitterionic materials (e.g. polybetaines/zwitterionic SAMs) iii) Amphiphilic materials (e.g. hyperbranched amphiphilic fluoropolymers)
28
New cards
What does it mean that non-fouling surfaces must be antiinflammatory and antithrombogenic?
Non-fouling surfaces are surfaces that resist adsorption. Thus, they would avoid the adhesion of proteins from the blood into the surface, not provoking the subsequent adhesion of platelets and the coagulation cascade. Similarly, by not allowing the adsorption of proteins, the surface would not have integrin-binding domains that can activate phagocytes and launch an inflammatory response.
29
New cards
What are the 4 major driving forces of adsorption?
Hydrophobic: entropy-driven process (release of “frozen waters” from the hydrophobic surfaces). A nonpolar hydrophobic surface usually exhibits significant protein adsorption and this contact often induces a denaturation of an amphiphilic protein molecule. Electrostatic: long range force. As most serum proteins are negatively charged in vivo, a negatively charged surface should be less favorable for protein adsorption. Van der Waals (vdW): short-range force. The superior protein resistance of PEGfunctionalized surfaces provides a repulsive force (steric hindrance effect) and significantly reduces the vdW interactions between protein and the biomaterial Hydrogen bonding: hydration properties of a surface render the proteins competition with water molecules for the surface less favourable (better non-fouling properties)
30
New cards
What are the two stages of protein-surface interactions?
Protein adsorption. Reversible: it can be desorpted.Irreversible protein denaturation
31
New cards
What are the general, non-specific characteristics of adsorption?
Surface hydrophobicity, Surface charge (though not completely separated from hydrophobicity effects) and Physical characteristics like surface roughness or how steric concerns, such as steric hinderance, lowers the rate of adsorption.
32
New cards
What are the main compounds that adsorbate to biomaterials?
At physiological conditions adsorbate on the biomaterial surface is composed primarily by ions, water and protein
33
New cards
What is surface tension?
Atoms at the surface of a material have asymmetric neighborhood and a higher energy. This excess energy is named surface tension
34
New cards
Where can the consequences of tissue-material incompatibility be seen?
The consequences of interactions may be localized to the vicinity of the material, often referred as the foreign body response, or they may be seen at some distance site, or they may be truly systemic
35
New cards
Are biocompatibility controlled and linear mechanisms?
No, in biocompatibility, an event may be triggered spontaneously, at any time, the effect of which can be powerfully amplified by one or more mechanisms, changing the whole nature of the response is a short space of time. Mechanisms of biocompatibility do not necessarily show a linear progression in time
36
New cards
What is biocompatibility?
The ability of a material to perform with an appropriate host response in a specific application. It is not a property of a material
37
New cards
What are conductive ECM structures?
They consist of a conductive bioink that is made from dECM\ cells, and multi-walled carbon nanotubes (MWCNTs)
38
New cards
What are the steps for FRESH extrusion bioprinting?
Preparation of gelatin bath for FRESH extrusion printing:
1. Dilution of gelatin and CaCl2 at 40 C
2. Gelation of gelatin in fridge overnight
3. Blending of cold gelatin to form gelatin microparticles
4. Centrifugation to remove insoluble gelatin and sediment slurry
5. Removal of foam and supernatant
6. Pouring of gelatin slurry into desired substrate and start 3D bioprinting!
1. Dilution of gelatin and CaCl2 at 40 C
2. Gelation of gelatin in fridge overnight
3. Blending of cold gelatin to form gelatin microparticles
4. Centrifugation to remove insoluble gelatin and sediment slurry
5. Removal of foam and supernatant
6. Pouring of gelatin slurry into desired substrate and start 3D bioprinting!
39
New cards
What is FRESH bioprinting? What are the main differences between this method and conventional bioprinting?
Conventional:
* High viscosity materials (above 500 kPa)
* Low water content
* Silicon and thermoplastics
FRESH:
* Low viscosity materials (below 500 kPa)
* High water content
* Cross-linking agents can be added to the gelatin bath
* Frequently used in the bioprinting of natural polymers
* High viscosity materials (above 500 kPa)
* Low water content
* Silicon and thermoplastics
FRESH:
* Low viscosity materials (below 500 kPa)
* High water content
* Cross-linking agents can be added to the gelatin bath
* Frequently used in the bioprinting of natural polymers
40
New cards
What is ionic cross-linkning?
This gelation mechanisms follows the “egg-box” model. In alginate, divalent ions interact simultaneously with the hydroxyl and carboxyl groups of the guluronic acid units present in the alginate chain forming a stable egg-box motif. If we increase the ion concentration, more polyguluronate sequences are cross-linked forming a hydrogel
41
New cards
What is Light-induced crosslinking?
Light (UV or visible) initiate and propagate a polymerisation reaction to crosslink a material. A water-soluble biocompatible photoinitiator needs to be added to the ink formulation (LAP, Irgacure 2959 or Eosin-Y) Typical from GelMA or ColMA
42
New cards
What is Temperature-induced crosslinking? It is typical of what compounds?
Changes in the temperature induce morphological changes in the arrangement of the molecular structure of the materials that lead to gelation Typical from collagen, gelatin and dECM Collagen needs to be printed at low temperatures (around 4oC), increasing the temperature to 37oC triggers the collagen fibrillogenesis process Temperature is applied to Collagen moleculesThe molecules form triple helixesFibers are form in a lateral and longitudinal until equilibrium is reached.
43
New cards
What is post-processing? When it happens in the printing process?
Bioinks based on natural polymers are often in liquid form and they have low viscosity due to their high water content. Therefore, 3D biopringing of hydrogel materials require gelation and crosslinking (otherwise the structures would collapse). Gelation and crosslinking usually takes place after printing, but can and sometimes it has to be performed between layers or during the deposition of the bioink
44
New cards
What is PGA?
Plyflycolic acid is a thermoplastic polymer with a high melting point and it is more acidic and hydrophilic than PLA. It is used as a surgical suture fibre due to its high mechanical and biocompatible properties. PGA requires precise control as it is highly sensitive to degradation and glycolic acid produced during its biodegradation may cause tissue damage
45
New cards
What is PLA?
Polylactic acid (PLA) possess readily available thermoplastic properties and high mechanical properties. Its molecular weight has a significant effect in biodegradability and high molecular weight PLA is likely to cause inflammation and infections in vivo, therefore, before 3D bioprinting it is vital to assess its properties and effects.
46
New cards
What is PCL?
Polycaprolactone is the most commonly used synthetic polymer in 3D bioprinting as it has superior printability due to its low melting temperature, it is biocompatible and biodegradable (it does not release toxic materials). It can be blended with other materials
47
New cards
What is alginate?
Anionic polysaccharide distributed in the cell walls of brown algae that has been extensively used as a wound dressing material. It polymerises in the presence of multivalent cations (Ca2+, Ba2+ etc). However, alginate has no-cell adhesive site.
48
New cards
What is dECM?
When cells are removed from a tissue, structural proteins specific from that particular tissue remain (collagen, glycosaminoglycans, elastin, fibrin etc.). Each tissue has its printability properties. dECM gelates with temperature (as its primary component is collagen).
49
New cards
What is gelatin? What is GelMA?
It is a type of protein obtained from collagen as a partially hydrolysed form and it is in liquid form at body temperature. For this reason it is usually mixed with other polymer materials. A chemically modified form, gelatin methacrylate (GelMA), can be photopolymerised and crosslinked in the presence of a photoinitiator and UV/visible light and it is more attractive than gelatin in 3D bioprinting.
50
New cards
What are collagen main characteristics?
Consists of mainly proline and glycine, with a triple-helix arrangement, and it is one of the most abundant proteins in the human body. Collagen type I is the most used in 3D bioprinting and it is regarded as the most promising material for this application. It crosslinks and gelates with temperature
51
New cards
Why are hydrogels the most common biomaterial used in 3D bioprinting?
Hydrogels are the most commonly used biomaterials for bioprinting because they recreate features of the natural extracellular matrix and allow for cell encapsulation in a hydrated and mechanically stable 3D environment
52
New cards
What are most commonly used materials used in bioprinting?
The most commonly used materials used in bioprinting include: Collagen - Gelatine methacrylate (GelMA) Decellularised extracellular matrix - Alginate
53
New cards
Advantages and disadvantages of extrusion-based bioprinting.
Advantages It is currently the best bioprinting method to create 3D structures. Very versatile technique in terms of materials and conditions where it can be used Inexpensive instrumentation required Disadvantages: Lower cellular viability due to high shear stress Low resolution Slow print speed
54
New cards
What is material jetting bioprinting? What are the most common techniques? What are their advantages and disadvantages?
Material jetting consist of the use of an actuator that precisely control the release of drops of bioink. The techniques are inkjet printing, and aerosol printing and poly jet the advantages are that it is the most clinically advanced bioprinting technique, and it can quickly generate skin grafts, But it has to have a high cell-density and also, it has to be a non very viscous fluid. Also, the bioink has to solidify after contact with the substrate.
55
New cards
What is lasser-asssted bioprinting? Whaare their advantages and dissadvantages?
It consist of the use of a laser to create bubbles that, when popped, will release the bioink in the target. It allows for the precise placing of cells on solid structures, but slow and wasteful. It cannot print layers easily.
56
New cards
What is stereolitography? What are its advantages and disadvantages?
A polymer is selectively polymerized through a laser (photopolymerization) It is the only microfabrication technique with microcellular resolution, and it allows the fabrication of very complex structures. However, there are not a lot of examples in the literature, and it needs the use of very expensive equipment. Also, the printing speed is very slow.
57
New cards
What are the three bioprinting stages?
Pre-processing: Cell selection and cultureHydrogel/scaffold selectionBioink preparationMorphological design Processing: Selection of bioprinting technique, and printing Post-processing: Further stabilization with cross-linking, sacrifical links, seeding of cells.
58
New cards
What are advantages and disadvantages of 3D biopriniting?
Advantages: No need for immunosupressant drugsNo chance of DNA rejectionDiminishing the use of animal modelsPersonalized use of drugsPersonalized using patient’s own cells Disadvantages: Not a lot of clinical success casesControversial use of stem cellsProbably more expensive than regular transplantNeed specialized workers
59
New cards
What are the functions of cells and biomaterials in 3D bioprinting?
Cells: tissue functionality Biomaterials: support, structure and signaling
60
New cards
What is bioprinting important?
It can supply de demand from organ transplants. 18 patients die each day, that is more thar 6000 a year. In europe, 48000 are on the waiting list for organ donations, and 6 are added every hour. Besides, bioengineered organs, if succesful, will not have problems with rejection or the need for immunosupression.
61
New cards
Name the 8 types of corrosions and their characteristics
Uniform attack: typical corrosion when a metal is in presence of a electrolytic solutionGalvanic corrosion: presented when two metals in close contact present a difference in potential, generating a galvanic reactionFretting corrosion: when two metals are too close together, they miromovements can cause the degradation of the passive layer and the rapid subsequent corrosioncrevice corrosion: creaves in the metal provoke areas deficient of oxygen that in turn provoke the aniodizartion of the area, generating a differeence in potenntial that end up in the genereation of creavesPit corrosion: related to creavation, micropits form in the metalintergranular corrosion: is a type of galvanic corrosion between the impurities presented as granules in the metalStress corrosion: when a metal is bended, a tensile stress is generated on its convex surface, and a compressive stress in the opposite one, making the former aniodic, generating a difference in potential that induces corrosion.
62
New cards
What are the main investigated corrosion mechanisms?
Passivity Adsorption Transpassivity
63
New cards
What are the 4 main characteristics expected in a passive film ?
* Non-porous
* fully cover the metal surface
* protect the metal from the exchange and movement of electrons and ions
* they can resist mechanical stress or abrasion, staying in the surface in place
* fully cover the metal surface
* protect the metal from the exchange and movement of electrons and ions
* they can resist mechanical stress or abrasion, staying in the surface in place
64
New cards
What is tha tfirst reaction that occurs in corrosion?
When a metal is in solution, the valence number of the metals increase, liberationg positively charged ions of the metal that can go away as free ions, or react with surrounding chemical compounds, to form oxydized metals, organometalliccompaunds, organochlorides, and others. These can stay dissolved in the aqueous phase or precipitate as a solid.
65
New cards
What are the two main corrosion mechanisms?
The thermodynamic forces, dependant on the energy of the chemical reaction, and the kinetic barriers, which limits the rate of the reaction.
66
New cards
How does bloodflow affect biomaterial?
For corrosive materials, the flow can interact with them, provoking the liberation of corrosive compunds and metallic ions. The greater the speed, the faster the rate of corrosion. For passive metals the flow will enhance the reduction of dissolved O2. The flow rate will not influence the rate of corrosion given the presence of the passive film.
67
New cards
What is the range of blood flow in the human body?
0.1 to 1 m/s
68
New cards
What is an acceptable corrosion degrade rate?
0.25 micrometers per year
69
New cards
What are examples of 5 toxic corrosive metals, and their effects on the body?
Niquel: generates dermatitis Cobalt: disturbs the central nervous system and provokes ulcers Chromium: inihibits iron absorption by the body, causing anemia Vanadium: it is highly toxic for the body in its elementary state Aluminum: provokes alzeimer and epileptic effects
70
New cards
What does biofunctionalization consists of?
Is the immobilization of biomolecules on the surface of biomaterials.
71
New cards
What are 6 examples of surface modification for ceramics?
Acid etching, laser treatment, ultraviolet treatment, sandblasting, calcium phosphate, biofunctionalization
72
New cards
What are the main characteristics of zirconian-based biomecial applications?
They show similar diffuse transmission and diffuse and specular reflection as teeth, making them perfect for dental implants.
73
New cards
What are tantalum’s main charactristics?
Is a strong, ductile biocompatible material with great corrosion resistance. Is mainly used for bone implantation as it has been shown that lasts for 8 to 12 years inside the body.
74
New cards
What are 4 examples of biomimetic coatings”?
Polyvinil acetate (PVA)Polilactic acid (PLA)Argininine-Glycine-Aspartic acid (RGD)Superhydrophobic compunds, with either long-chain fatty acids or organosilanes (C-Si compunds)
75
New cards
\____ \______ has been successfully usedto produce - on AZ31 Mg alloy. It presented a \____ surface and improved \___ \________
Aerosol deposition has been successfully used to produce HA Chitosan on AZ31 Mg alloy. It presented a rugged surface and improved corrosion resistance.
76
New cards
What are biomemitic coatings?
They are coatings that can be produced by mimicking how they are done in nature or that they mimic the characteristics of natural surfaces itself.
77
New cards
Why is Mg good for bone implants?
It has a young modulus similar of bone, and a good tensile strenght. This implies it doesn’t have the “bone shielding effect“, in which the mechanical properties of the implant shields the bone from the normal stresses it should receive, hindering its growth.
78
New cards
How is MgO coating produced?
It starts by the anodic electrodeposition in KOH solutions and then the subsequent annealing and calcination for the formation of the film.
79
New cards
Give 2 examples of Ca-P coatings
Ocatacalcium phosphate (OCP) and **β-**tricalcium phosphate ( β-TCP)
80
New cards
What are the main treatments that can be done to Mg applications for the increase of corrosion resistance?
Purification: the presence of Ni, Cu and Fe impurities greatly reduces its corrosion resistance. Thus, the elimination of these impurities can augment it.Alloying: Mg, Zn and Ca are all metals that can be added to Mg to form an anti corrosion alloy, given the properties of this metal to form inert harmless intermetallic compounds with the aforementioned impuritiesSurface coating: the coating with Ca phospate and the treatment with proteins can also be used.
81
New cards
What are the main applications of magnesium applications? What are the expected timeframes for which the metal will have not lost its mechanical properties due to degradation for each of those?
Vascular stents and bone implants. For the former is expected from them to retain their mechanical properties for up to 6 months, and for the latter 3 months.
82
New cards
What are the possible consequences for the early degradation of magnesium-based applications?
The early failure of a stent could imply a thrombosis or stenosis, while the failure of a bone implant could imply the liberation of gases that do not help bone regeneration, and the loss of strength in the implant. However, the increased pH can also induce more bone regeneration. But this also provokes the increase of hemolysis beyond the recommended rate.
83
New cards
What are the advantages and disadvantages of magnesium-based implants?
They have a big load capacity, and have been use as vasodilators. However, they have low corrosion resistance around Cl, thus, in this environments they can show earlier degradation and failure, excessive hydrogen production and thus, gas cavities and inflammation, and a high pH increase.
84
New cards
What effect has calcium?
It can activate fibrin polymerization and the activation of platelets.
85
New cards
What is PDMSUr? What is capable of?
Polidimethylsiloxanehydroxyurethane is a molecule capable to strongly attach to other metals or other inorganic materials and create an hydrophobic layer on top of it.
86
New cards
What are catecholic moieties?
Chemical groups that contain a benzene ring with a hydroxyl group and an adjacent carbonyl group. They are important in the formation of melanin and other pigments, as well as in the degradation of neurotransmitters like dopamine and norepinephrine.
87
New cards
What elements are considered safe for the fabrication of metallic alloys for biomedical applications?
Selenium, Zirconium, Molybdenium, tantalum, niobium
88
New cards
What elements can cause an allergic reaction if used in a biomedicalmetal alloy?
Nickel, chromium, cobalt.
89
New cards
What are the different types of coatings for metals?
Conversion and Deposited. Conversion arise in complex interactions between the metal dissolution and precipitation, during treatment in aqueous solution. Tipically the metallic substrate is converted to an oxide layer, inorganic with ceramic-like properties. Deposited coatings can be divided into metal and organics and inorganic based coatings. Mostly though, they are organic.
90
New cards
Give examples of the different types of stainless steel and its applications
Austenitic: hip replacement, short-term implants. Ferritic: screws, fasteners, cannulas, guide pins. Martensitic: forceps, scalpels, chisels, dental burs, root elevator
91
New cards
What is a limitation of stainless steel?
It is not resistant to corrosion, thus, it is limited to temporary applications only.
92
New cards
What are the keys characteristics of biomaterials?
They are immune acceptable, and they minimize the inflammatory response. They have biomechanical properties to maintain tissue growth and integration They should be biodegradable or designed to be expelled from the system, and engineered to degrade at the same rate that allows for tissue growth. They have microtopography that allows for cell-material adhesion They are porous, allowing the anchorage of cells, maximizing migration, allowing the secretion of the ECM, vascularization, and the circulation of medium and other fluids. They have an adhesion-favorable surface. This can be achieved through physical adsorption or chemical modification.
93
New cards
What are the different goals that surface modification may have?
Improve adhesionImprove wettabilityImprove protein adsorptionProtect the human bodyImprove blood compatibilityImprove corrosion resistanceImprove lubricityImprove hardness
94
New cards
Why biomaterials surface matter?
Biomaterials are materials that, used alone or as part of a complex system, interact with the components of living systems to direct a therapeutic or diagnostic procedure.
95
New cards
For polymers, what phase separation consists of?
Plastic is dissolved at high temperature in a solute, and then cooled. The fraction of the solvent will indicate if the solid phase, after cooled the separated from the loquid phase, if it will remain powdered, a scaffold, or a foam.
96
New cards
What foaming consists of?
It is the process of using an inert gas, usually CO2 or N2, for the introduction of pores in a polymer. Different polymers can be combined, and variations of the process can induce different processes on the result. Usually the result has a uniform surface and the pores are not interconnected (called non-percolated pores in the literature). Thus, they have to be plasma treated or pulse-ultrasounded to break the walls between the pores.
97
New cards
What are the principal characteristics of electrospinning?
A charged polymer is ejected into an oppositely charged surface.Multiple polymers can be combinedExist control over the fiber diameter and scaffold structure.
98
New cards
Give an example of a biomedical application of casted hydrogels made with cast molding
Contact and intraocular lenses
99
New cards
How does blow molding work for polymers?
The molten plastic is extruded to the center of a cold mold, which has a blow pin and the plastic given the shape of the mold thanks to the air blown. Instead of a mold, the blown polymer can create a bubble that when guided and pinched with rolls can produce plastic films.
100
New cards
How does extrusion works for polymers?
Plastic pellets are put in a hopper where they are fed through the barrel which encapsulates a rotating screw that drives the pellets through it while they are simultaneously being heated, converting it to molten plastic, and finally shaped and extruded at the end.