Mastering Chemistry Chapter 9 Modified
1/31
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
32 Terms
A certain AB4 molecule has a seesaw shape. From which of the fundamental geometries could you remove one or more atoms to create a molecule having this seesaw shape?
o o
| /
O - o
|
o
trigonal bipyramidal
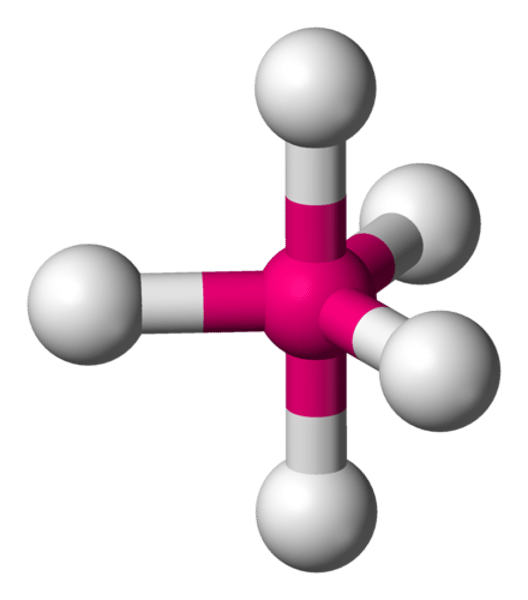
What is the molecular geometry of a molecule with 2 outer atoms and 1 lone pair on the central atom?
Bent
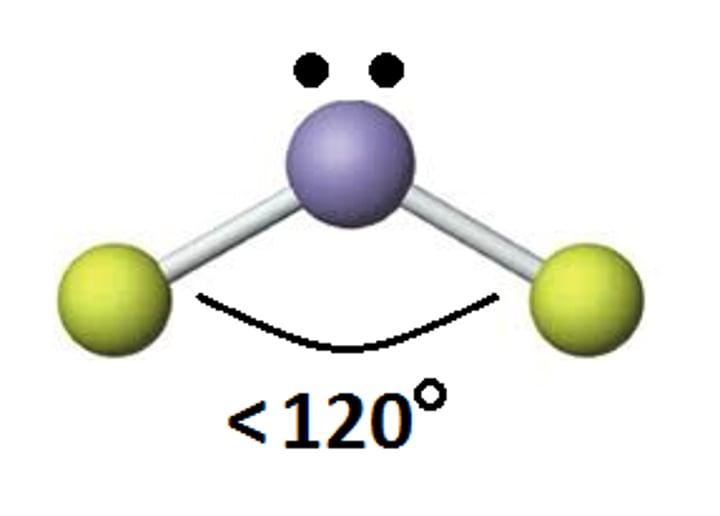
What is the molecular geometry of a molecule with 2 lone pairs on the central atoms?
Square planar
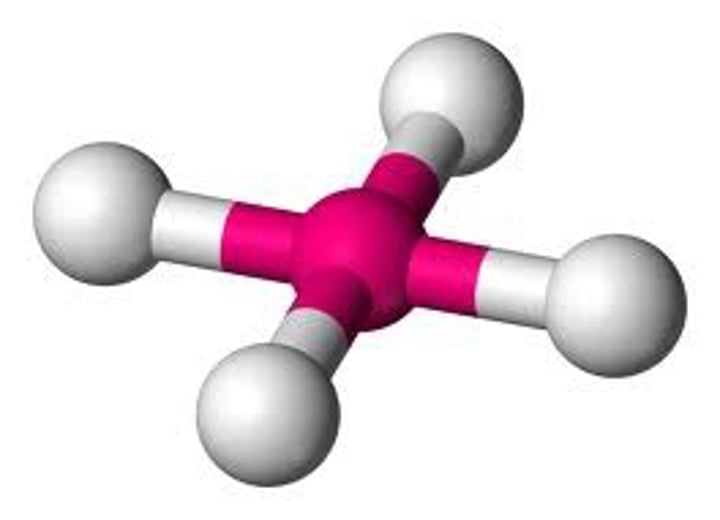
What is the molecular geometry of a molecule with 2 outer atoms and 3 lone pairs on the central atom?
Linear
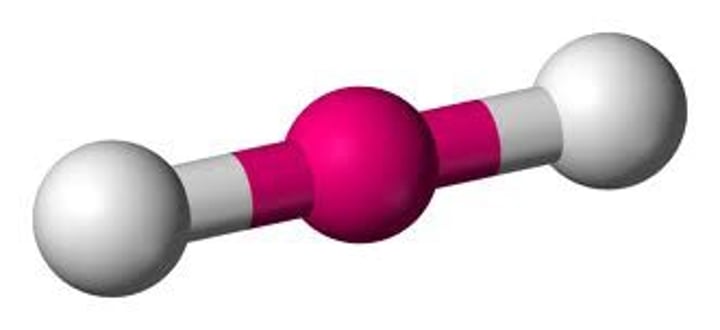
The atoms of the compound methylhydrazine, CH6N2, (rocket propellant), are connected like:
H
|
H --- C --- H
|
N --- N --- H
| |
H H
What do you predict for the ideal values of the C-N-N and H-N-H angles, respectively?
109.5 & 109.5
PF3Br2 is a nonpolar molecule. Determine the F-P-F bond angle, Br-P-Br, F-P-Br
120, 180, 90
Which statement best describes the polarity of CHCl3?
the molecule is always polar
Which statement best describes the polarity of SF4Br2?
Depending on the arrangement of outer atoms, this molecule could be polar or nonpolar
Predict whether each of the following molecules are polar or nonpolar: XeF4, CH3Br, GaH3, NH3, CCl4, SF4
Polar- NH3, CH3Br, SF4
Nonpolar- XeF4, CCl4, GaH3
For which of the following molecules or ions does the following description apply: 'The bonding can be explained using a set of sp^2 hybrid orbitals with one of the hybrid orbitals holding a nonbonding pair of electrons."
O3
How many electrons are in the π system of the ozone molecule, O3?
4
Which ABn molecule is a trigonal pyramidal?
NH3
In addition to a tetrahedral, another common shape for AB4 molecules is a square planar. All 5 atoms lies in the same plane with the B atoms at the corners of a square and the A atom at the center of the sphere. Which shape could lead to a square-planar shape upon removal of one or more atoms?
octahedral
In a space filling model, what determines the relative size of the spheres?
the atomic radii
The molecular geometry of the CS2 molecule is ______
linear
Of the following species, _______ will have bond angles of 120
BCl3
The molecular geometry of the CHCl3 molecule is __________
tetrahedral
Using the VSEPR model, the electron domain geometry of the central atom in BF3 is ___________
trigonal planar
The F-B-F bond angle in the BF3 molecule is _______
120
The molecular geometry of the H3O^+ ion is _________
triagonal pyramidal
PCl5 has _____ electron domains and a ________ molecular arrangement
5, triagonal bipyramidal
An AB6 molecule has no pone pairs of electrons on the A atom. What is its molecular geometry?
octahedral
An AB4 molecule has one lone pair of electrons on the A atom(in addition to the 4 B atoms). What is the electron domain geometry around the A atom? Also, predict the molecular geometry.
triagonal bypyramidal, seesaw
The hybridization of the A atom in CO2 is ______
sp
The angles between sp^2 orbitals are _____
120
There are ___ σ and ____ π bonds in H-C=C-H molecule
3,2
True or False: Electrons in core orbitals contribute to atom bonding
False
A typical double bond consists of ___________________________
2 shared electron pairs
Stronger and shorter than a single bond
1 σ bond and 1 π bond
Imparts rigidity to a molecule
A typical triple bond _______
consists of 1 σ bond and 2 π bonds
What is the value of the σ ls^* MO wave function at the nodal plane?
0
Molecule orbital theory correctly predicts paramagnetism of oxygen gas, O2. This is because ________________
There are 2 unpaired electrons in the MO electron configuration of O2
Molecule orbital theory correctly predicts diamagnetism of fluorine gas, F2. This is because __________________
All electrons in the MO electron configuration of F2 are paired