Chap 5B - Enthalpy
1/8
Earn XP
Description and Tags
Experimental determination
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
9 Terms
Explain how a calorimeter is used to determine heat change
Polystyrene is an excellent insulator so heat loss from the sides is minimised
The lid also helps to minimise heat exchange with the surrounding air
The polystyrene cup is often supported in a 250 cm3 beaker or using a retort stand, to prevent toppling under the weight of the thermometer
The insulation can be improved by nesting two cups
The temperature changes are often small, so for accurate work the thermometer should have a precision of ±0.1C
Just state some important stuff to take note of when calculating H
If question says refer to Data Booklet, use the value c = 4.18 J g–1 K–1
Sometimes, heat capacity, C, in J K–1, is given where C = mc
Answering: heat is evolved or absorbed
Temperature change = MUST be positive
If the experiment involves dissolving a solid in water:
Ignore mass of solute in calculating mass of solution
Assume density of solution to be same as water (1.00 g cm-3)
To find H for a reaction:
Write balanced equation with state symbols
Find the amount of limiting reagent used
Calculate heat evolved or absorbed using mcT or VcT
Calculate H using heat evolved or absorbed ÷ amount of limiting reagent
Convert heat evolved or absorbed to enthalpy change per mole of the limiting reagent
H is negative for a rise in temperature (exo)
H is positive for a drop in temperature (endo)
Include a sign (+/−) for H
Describe limitations of calculations
For exothermic reactions: heat loss to surroundings during the experiment
Experimental enthalpy change calculated will be less exothermic than expected
For endothermic reactions: not all heat gain from surroundings during the experiment
Experimental enthalpy change calculated will be less endothermic than expected
Describe bomb calorimeter
A known mass of the substance (whose Hc is to be determined) is burnt in excess oxygen in a bomb calorimeter
The heat released is transferred to a known mass of water in the calorimeter
The rise in temperature of the water is then measured
Allowance is made for the heat capacity of the bomb calorimeter and the heat evolved in burning the iron ignition wire used to ignite the substance
Features:
Benzoic acid is often used in the crucible as the ‘bomb’: stable solid that is easy to be handled
High pressure of O2 is used in calorimeter: EC not measured under standard conditions so SEC of combustion calculated is slightly different from enthalpy change calculated
Describe thermometric titration
Enthalpy change may be determined by measuring the temperature change during a modified titration where fixed volumes of reagent from the burette are dispensed at regular intervals to the other reagent
The resultant highest/lowest temperature is then recorded
Describe dissolution of ionic solid using LE and hydration
Breaking up the solid ionic lattice to form isolated gaseous ions
The ions are separated from the ionic lattice
A quantity of heat equivalent to the lattice energy is absorbed to break the solid ionic lattice into separate gaseous ions
Process is endothermic (overcoming ionic bonding) and the enthalpy change is ‘–LE’
MX(s) → M+ (g) + X– (g) H = –LE
Hydration of the gaseous ions
The free gaseous ions are then attracted by the polar water molecules (ion-dipole attractions)
The attraction between the oppositely charged ions and the polar water molecules results in a release of hydration energy
Process is exothermic (forming ion-dipole interactions between ion and water)
State the equation relating LE, hyd and sol

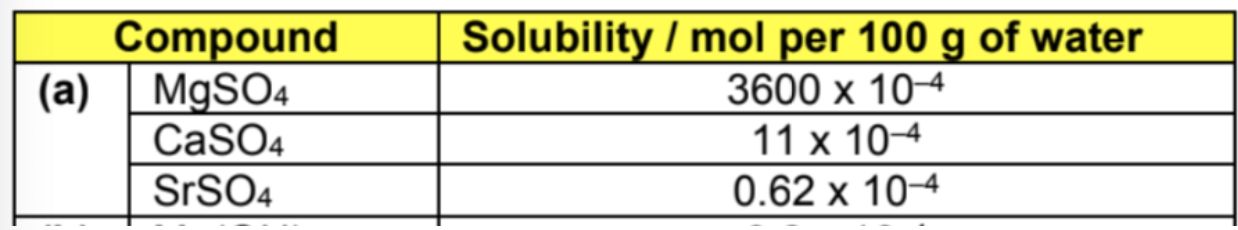
Describe solubility of the following substance using LE, hydation and sol
LE
Down the group, q+ and q− remain constant throughout
As the SO42– (r−) is a very large anion, the sum of r+ and r– would show little changes down the group since the cations are much smaller than it -> lattice energy remains relatively constant from MgSO4 to SrSO4
Hhyd
There is no change to Hhyd (anion) down the group since the sulfate anion is unchanged
As for the cations, the ionic radius increases down the group but charge remains constant at +2, the Hhyd (cation) would therefore become less exothermic down the group
Overall, the sum [Hhyd (cation) + Hhyd (anion)] becomes less exothermic down the group
Hsol
Overall, solubility of the group 2 sulfates decreases down the group since the hydration energy becomes increasingly unable to compensate for the very exothermic lattice energy, leading to more endothermic Hsol

Describe solubility of the following substance using LE, hydation and sol
LE
Since r– is very small, lattice energy is sensitive to a change in r+
Thus lattice energy becomes less exothermic with increasing cationic radius down the group
Hhyd (always negative!)
Hhyd (cation) becomes less exothermic on descending down the group
This effect is largely masked by the large magnitude of the Hhyd (anion), since OH− has a very small r− as compared to the cations
Thus, the sum [Hhyd (cation) + Hhyd (anion)] is relatively constant
Hsol
Since there is a decrease in magnitude of lattice energy and sum [Hhyd (cation) + Hhyd (anion)] is relatively constant, the hydroxides become more soluble down the group, as shown by a more exothermic Hsol (-LE is positive as LE is negative, HHyd is negative → if LE is more positive → Hsol is more negative)