Topic 15: Transition Metals
1/50
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
51 Terms
Transition metals
Transition metals are d-block elements that can form one or more stable ions with incompletely filled d-orbitals.
d-block elements that are not transition metals
Scandium and zinc.
How transition metals form ions
Transition metals lose electrons from their 4s orbital first, then their 3d orbitals, as the 4s subshell has the highest energy.
Electron configuration of Cu2+
1s22s22p63s23p63d9
Electron configuration of Cr6+
1s22s22p63s23p6
Why transition metals show variable oxidation number
The 3d and 4s subshells are very close in energy.
This allows transition metals to first lose electrons from their 4s subshell and then also lose more electrons from the d-subshell without a large increase in energy required, so the successive ionisation energies increase gradually.
Ligand
A ligand is a species that uses a lone pair of electrons to form a dative covalent bond with a central metal ion.
Complex
A complex is a central metal ion surrounded by ligands, bonded to the metal ion with dative bonds.
A complex with a charge is a complex ion.
Coordination number
The number of dative covalent bonds in a complex is its coordination number.
Why the solutions of aqueous ions of transition metals are coloured
The d-subshell of the ions of transition metals are incompletely filled, so when water ligands attach to the ion, the d-orbitals split into two different energy levels.
The electrons in the d-orbitals absorb energy from the visible spectrum, causing them to move from a lower energy d-orbital to a higher energy d-orbital.
The remaining colour of light that is not absorbed is transmitted and seen as colour.
Why zinc ions do not form coloured solutions
Zn2+ ions have a full 3d subshell.
This means electrons cannot move between 3d orbitals.
Why aluminium ions do not form coloured solutions
Al3+ ions have no electrons in their 3d subshell.
This means there are no d-orbital electrons that can absorb energy and move between 3d orbitals.
Shapes of different complexes
When the coordination number is 6, the complex has an octahedral shape.
When the coordination number is 4, the complex has a tetrahedral shape or sometimes a square planar shape.
When the coordination number is 2, the complex has a linear shape.
Monodentate ligands
A ligand that forms one dative bond with a metal ion.
Examples are H2O, OH-, and NH3, as they all only have one atom with a lone pair of electrons.
Ligands that form octahedral complexes
NH3, OH-, and NH3, as they are relatively small and can fit around the central ion.
Ligands that form tetrahedral complexes
Cl- ions, as they are relatively large, so more than four cannot fit around the central ion.
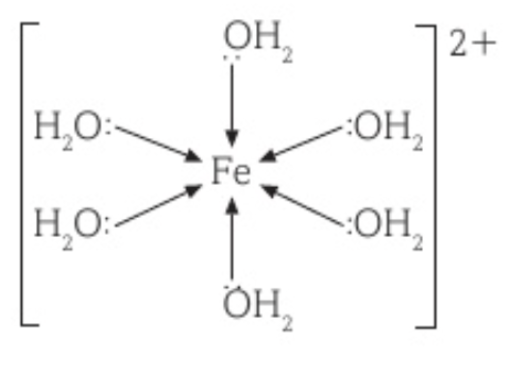
Complex ion of Fe2+ with H2O
[Fe(H2O)6]2+
Coordination number is 6.
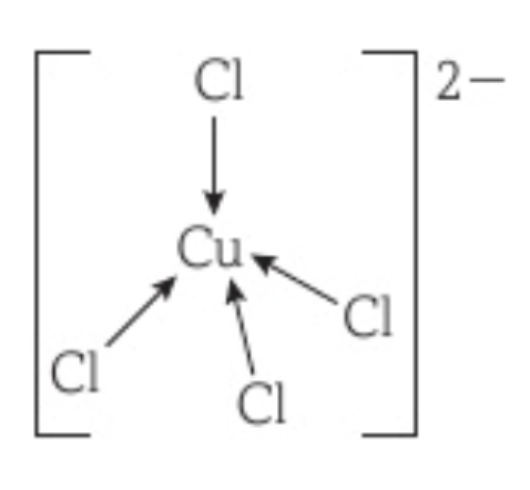
Complex ion of Cu2+ with Cl-
[CuCl4]2-
Coordination number is 4 and is tetrahedral.
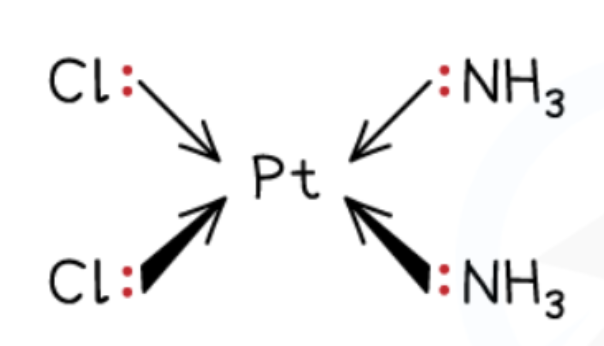
Cis-platin complex
[Pt(NH3)2Cl2]
Consists of Pt2+ ion, two ammonia ligands, and two Cl- ion ligands.
The cis- prefix indicates that identical ligands are next to each other.
The complex has a square planar shape with bond angles of 90o.
Usage of cis-platin
Cis-platin is used to treat cancer.
When cis-platin enters the cell, it loses its chloride ligands, which are replaced by water molecules.
Cis-platin becomes a highly reactive, positively charged ion.
The water ligands are removed and replaced by dative covalent bonds to two adjacent guanine bases on the same strand of DNA, which is made possible by the two water ligands being on the same side of the complex.
This disrupts the DNA structure and stops the cancer cell from dividing.
Trans-platin is not able to be used as cancer treatment because the water ligands are on opposite sides of the complex, so they cannot form dative covalent bonds with adjacent guanine bases on the same strand of DNA.
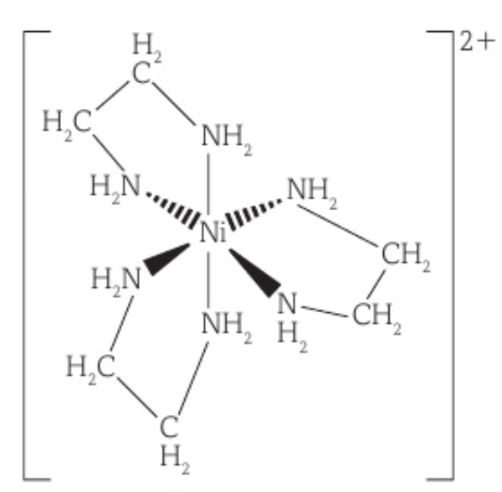
Bidentate ligands
A ligand that forms two dative bonds with a metal ion.
An example is NH2CH2CH2NH2, which uses the lone pair of electrons on each nitrogen atom to form two dative bonds with the metal ion. It is sometimes abbreviated as ‘en’.
Three NH2CH2CH2NH2 ligands bond with a metal ion to form an octahedral shape and a complex ion with a coordination number of 6.
Multidentate ligands
A ligand that forms several dative bonds with a metal ion.
An example is EDTA4-, which is formed when EDTA’s COOH groups each lose a H+ ion. It can form 6 dative bonds with a metal ion, four with its lone electron pairs on the negative oxygen of its COO- groups and 2 with the lone electron pairs on its nitrogen atoms.
One EDTA4- ligand bonds with a metal ion to form an octahedral shape and a complex ion with a coordination number of 6.
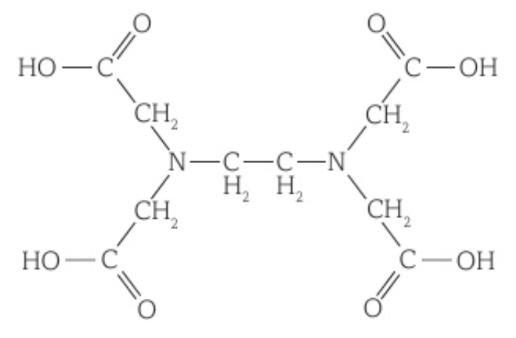
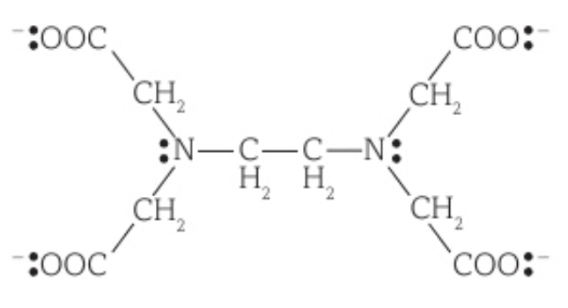
Substitution of a monodentate ligand by a bidentate or multidentate ligand
The substitution of a monodentate ligand by a bidentate or multidentate ligand creates a more stable complex ion because the reaction has a large positive ΔSsystem, meaning the system goes from a less stable state to a more stable state.
For example, [Cu(H2O)6]2+ + EDTA4- → [Cu(EDTA)]2- + 6H2O, shows that ΔSsystem is positive, as 2 moles of reactants forms 7 moles of products, meaning that there is an increase in disorder.
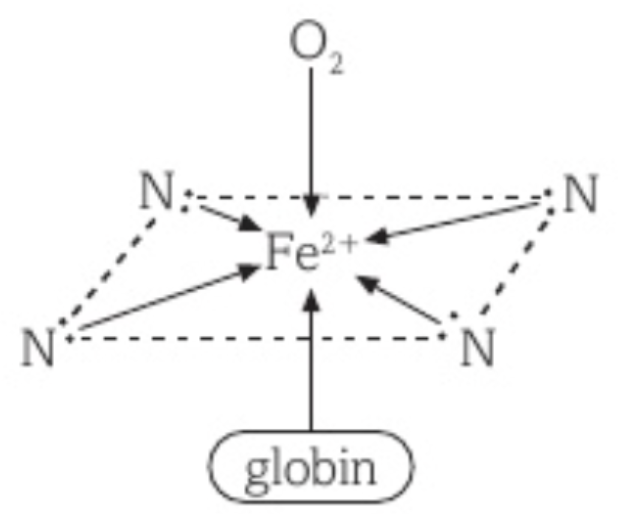
Haemoglobin
Haemoglobin is an iron (II) complex containing a multidentate ligand and 4 Fe2+ ions.
The largest part of haemoglobin is the protein, globin, which contains four haem groups.
Each haem group contains 4 nitrogen atoms, and each haem group forms four dative bonds, using their four nitrogen atoms, to each Fe2+ ion.
Another dative bond forms between the globin and each Fe2+ ion.
When haemoglobin carries oxygen, the oxygen molecule acts as a ligand, using one of its lone pairs of electrons to form a dative bond with the Fe2+ ion.
Carbon monoxide and haemoglobin
Carbon monoxide acts as a ligand by using its carbon atom’s lone pair of electrons to form a dative bond with a metal ion.
The strength of the dative bond between Fe2+ and carbon monoxide is a lot stronger than the strength of the dative bond between oxygen and Fe2+, so carbon monoxide readily replaces the oxygen from haemoglobin, causing a ligand exchange reaction.
This dative bond is so strong that the reaction is irreversible.
Causes of colour changes in transition metal ions
A change in oxidation number of the ion may change the colour of the ion.
When a metal ion has a higher oxidation number, there is a stronger electrostatic attraction to the ligands, causing the d-orbitals to be split further apart, creating a larger energy gap.
A change in ligand of the ion may change the colour of the complex ion.
The greater the charge density of a ligand, the greater the electrostatic attraction to the metal ion, causing the d-orbitals to be split further apart, creating a larger energy gap.
A change in the coordination number of the complex ion may change the colour of the complex ion.
A higher coordination number of the same ligands will cause the d-orbitals to be split further apart, causing a larger energy gap.
Reaction of Cu2+ (aq) with aqueous sodium hydroxide
[Cu(H2O)6]2+ + 2OH- → [Cu(H2O)4(OH)2] + 2H2O
A blue solution forms a blue precipitate.
Two of the water ligands transfer a H+ ion to each OH- ion, so this is an acid-base reaction.
There is no change if excess NaOH (aq) is added.
Reaction of Cu2+ (aq) with aqueous ammonia
[Cu(H2O)6]2+ + 2NH3 → [Cu(H2O)4(OH)2] + 2NH4+
Two of the water ligands transfer a H+ ion to each ammonia molecule, so this is an acid-base reaction.
A blue solution forms a blue precipitate
If excess aqueous ammonia is added, the precipitate dissolves and the solution turns dark-blue: [Cu(H2O)4(OH)2] + 4NH3 → [Cu(NH3)4(H2O)2]2+ + 2H2O + 2OH-, which is a ligand exchange reaction.
The overall equation when excess aqueous ammonia is added is [Cu(H2O)6]2+ + 4NH3 → [Cu(NH3)4(H2O)2]2+ + 4H2O
Reaction of Fe2+ (aq) with aqueous sodium hydroxide
[Fe(H2O)6]2+ + 2OH- → [Fe(H2O)4(OH)2] + 2H2O
Two of the water ligands transfer a H+ ion to each OH- ion, so this is an acid-base reaction.
A green solution forms a green precipitate.
There is no change if excess NaOH (aq) is added.
Reaction of Fe2+ (aq) with aqueous ammonia
[Fe(H2O)6]2+ + 2NH3 → [Fe(H2O)4(OH)2] + 2NH4+
Two of the water ligands transfer a H+ ion to each ammonia molecule, so this is an acid-base reaction.
A green solution forms a green precipitate.
There is no change if excess aqueous ammonia is added.
Reaction of Fe3+ (aq) with aqueous sodium hydroxide
[Fe(H2O)6]3+ + 3OH- → [Fe(H2O)3(OH)3] + 3H2O
Three of the water ligands transfer a H+ ion to each OH- ion, so this is an acid-base reaction.
A yellow solution forms a brown precipitate.
There is no change if excess NaOH (aq) is added.
Reaction of Fe3+ (aq) with aqueous ammonia
[Fe(H2O)6]3+ + 3NH3 → [Fe(H2O)3(OH)3] + 3NH4+
Three of the water ligands transfer a H+ ion to each ammonia molecule, so this is an acid-base reaction.
A yellow-brown solution forms a brown precipitate.
There is no change if excess aqueous ammonia is added.
Reaction of Co2+ (aq) with aqueous sodium hydroxide
[Co(H2O)6]2+ + 2OH- → [Co(H2O)4(OH)2] + 2H2O
Two of the water ligands transfer a H+ ion to each OH- ion, so this is an acid-base reaction.
A pink solution forms a blue precipitate.
There is no change if excess NaOH (aq) is added.
Reaction of Co2+ (aq) with aqueous ammonia
[Co(H2O)6]2+ + 2NH3 → [Co(H2O)4(OH)2] + 2NH4+
Two of the water ligands transfer a H+ ion to each ammonia molecule, so this is an acid-base reaction.
A pink solution forms a blue precipitate.
If excess aqueous ammonia is added, the precipitate dissolves and forms a yellow solution: [Co(H2O)4(OH)2] + 6NH3 → [Co(NH3)6]2+ + 4H2O + 2OH-, which is a ligand exchange reaction.
The overall equation when excess aqueous ammonia is added is [Co(H2O)6]2+ + 6NH3 → [Co(NH3)6]2+ + 6H2O
Reaction of Cr3+ (aq) with aqueous sodium hydroxide
[Cr(H2O)6]3+ + 3OH- → [Cr(H2O)3(OH)3] + 3H2O
Three of the water ligands transfer a H+ ion to each OH- ion, so this is an acid-base reaction.
A green solution forms a green precipitate.
If excess aqueous sodium hydroxide is added, the precipitate dissolves to form a green solution: [Cr(H2O)3(OH)3] + 3OH- → [Cr(OH)6]3- + 3H2O
Three of the water ligands transfer a further three H+ ions to the OH- ions, so this is an acid-base reaction.
Reaction of Cr3+ (aq) with aqueous ammonia
[Cr(H2O)6]3+ + 3NH3 → [Cr(H2O)3(OH)3] + 3NH4+
Three of the water ligands transfer a H+ ion to each ammonia molecule, so this is an acid-base reaction.
A green solution forms a green precipitate.
If excess aqueous ammonia is added, the precipitate dissolves to form a violet solution: [Cr(H2O)3(OH)3] + 6NH3 → [Cr(NH3)6]3+ + 3H2O + 3OH-, which is a ligand exchange reaction.
The overall equation when excess aqueous ammonia is added is [Cr(H2O)6]3+ + 6NH3 → [Cr(NH3)6]3+ + 6H2O
Formation of [CuCl4]2- from [Cu(H2O)6]2+
[Cu(H2O)6]2+ + 4Cl- → [CuCl4]2- + 6H2O
This is a ligand exchange reaction.
The colour of the solution changes from blue to yellow.
Formation of [CoCl4]2- from [Co(H2O)6]2+
[Co(H2O)6]2+ + 4Cl- → [CuCl4]2- + 6H2O
This is a ligand exchange reaction.
The colour of the solution changes from pink to blue.
Substitution of small, uncharged ligands by larger, charged ligands
This ligand exchange reaction often results in a change in coordination number, as less of the larger, charged ligands can fit around the central metal ion, as there is more electrostatic repulsion between them and they are large.
Amphoteric behaviour of transition metal hydroxides with chromium (III) hydroxide as an example
Transition metal hydroxides can both accept H+ ions and donate them, so they are amphoteric.
[Cr(H2O)3(OH)3] + 3H+ → [Cr(H2O)6]3+
[Cr(H2O)3(OH)3] + 3OH- → [Cr(OH)6]3- + 3H2O
Equilibrium between chromate (VI) and dichromate (VI) ions
2CrO42- + 2H+ ⇌ Cr2O72- + H2O
When the concentration of H+ increases and the solution becomes more acidic, C2O72- forms, causing an orange colour.
When the concentration of OH- increases and the solution becomes more basic, 2CrO42- forms, causing a yellow colour.
Reaction of dichromate (VI) ions with zinc
In acidic conditions, zinc reduces dichromate (VI) ions first to Cr3+ ions and then to Cr2+ ions.
This is made possible because of the E⦵ values of the relevant reactions.
Reaction of Cr3+ ions with hydrogen peroxide
In alkaline conditions, hydrogen peroxide (H2O2) oxidises Cr3+ ions to dichromate (VI) ions.
This is made possible because of the E⦵ values of the relevant reactions.
Vanadium oxidation state colours of solutions
+2: purple
+3: green
+4: blue
+5: yellow
Heterogenous catalyst
A catalyst in a different state to the reactants.
They are often solids used in reactions involving gases, providing a surface for the reactant gas molecules to adsorb onto. The reactant molecules adsorb onto the active sites of the catalyst. Bonds between the catalyst and the reactant molecules are formed, weakening the bonds between the atoms of the reactant molecules, allowing a reaction to take place between the reactant molecules on the surface of the catalyst. The product then desorbs from the surface and more reactant molecules take their place.
Homogenous catalyst
A catalyst in the same state as the reactants.
The catalysed reaction proceeds via an intermediate species.
Usage of transition metals as catalysts
Transition metals and their compounds can act as both heterogeneous and homogeneous catalysts.
Transition metals and their compounds can act as catalysts because they have variable oxidation states, allowing them to change their oxidation state back and forth as they react with the reactants of the reaction.
How vanadium (V) oxide is used as a catalyst in the contact process
The contact process produces sulfuric acid.
The reaction of sulfur dioxide with oxygen to form sulfur trioxide is catalysed by vanadium (V) oxide: 2SO2 + O2 ⇌ 2SO3.
Vanadium (V) oxide is reduced by sulfur dioxide into vanadium (IV) oxide to form sulfur trioxide: SO2 + V2O5 → V2O4 + SO3
Vanadium (IV) oxide is the intermediate species.
The vanadium (IV) oxide then reacts when oxygen to reform vanadium (V) oxide, allowing it to fulfill its role as a catalyst: 2V2O4 + O2 → 2V2O5
Catalytic converter
A catalytic converter reduces carbon monoxide and nitrogen monoxide emissions from combustion engines.
The CO and NO molecules adsorb onto the surface of the catalyst.
The catalyst weakens the bonds between the atoms of the molecules, allowing them to react: 2CO + 2NO → 2CO2 + N2
The nitrogen and carbon dioxide products then desorb from the surface of the catalyst so more reactant molecules can take their place.
Reaction between I- and S2O82- ions
S2O82- ions oxidise I- ions: 2I- + S2O82- → I2 + 2SO42-
The reaction is slow at room temperature because the ions repel each other.
The reaction is catalysed by Fe2+ ions.
All the reactants and the catalyst are in the aqueous phase, so it is homogeneous catalysis.
When catalysed, Fe2+ reduce S2O82- ions, which are attracted to each other, to produce the intermediate species Fe3+: S2O82- + 2Fe2+ → 2SO42- + 2Fe3+
The Fe3+ ions oxidise I- ions, which are attracted to each other: 2Fe3+ + 2I- → 2Fe2+ + I2
This reforms the Fe3+, allowing it to fulfill its role as a catalyst.
Reaction between manganate (VII) and ethanedioate ions
Manganate (VII) ions oxidise ethanedioate ions: 2MnO4- + 5C2O42- → 2Mn2+ + 5CO2 + 8H2O
The initial rate of reaction is low because the ions repel each other.
Mn2+ autocatalyses the reaction, so the rate of reaction increases as the reaction proceeds.
Mn2+ is oxidised to Mn3+, which is the intermediate species, when it reacts with MnO4-: Mn2+ + MnO4- + 8H+ → 5Mn3+ + 4H2O
The Mn3+ ions are then reduced back to Mn2+ when they react with C2O42-: 2Mn3+ + C2O42- → 2CO2 + 2Mn2+
This reforms the Mn2+, allowing it to fulfill its role as a catalyst.