chemistry
1/17
Earn XP
Description and Tags
Volumetric analysis
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
18 Terms
Define volumetric analysis
Va is the versatile form of quantitive chemical analysis. the purpose is to use An accurately known volume and conc of one sol to find the accurate conc of 2nd sol
tools used in va
conical flask
Burette
pipette
retort stand
white tile
types of titrations
acid-base
complexometric
redox
precipitation
Analyte
substance being analysed
titrant
solution in the burette
moles formula
mass/molar mass
what is molarity
the conc of a solution in moles/litre (dm-3)
1dm-3 = ?
1000cm3
what is a titration
a procedure that measures the vol of one reagent required to react with a measured mass or vol or another reagent.
what’s a titration used for
to determine the amount of acid/base present in a sample
why is an indicator used?
to signal the endpoint, when the stoichiometric amounts of acid and base are present
function of a burette?
used to deliver the titrant into an erlenmeyer (conical) flask
define titration
an analytical procedure whereby we react a known volume of solution of known conc with a vol of a sol of unknown conc.
chemical formula of titration
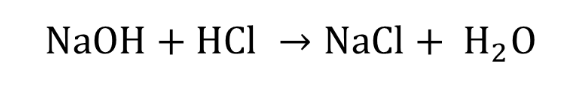
define standard solution
sol of known conc
define primary standard
a standard sol which has been prepared by accurately weighing out a pure compound and using it to make a solution of known volume (made in vol flask)
define secondary standard
a solution standardised against a primary standard