GEN CHEM 1 | SA3 Reviewer
1/145
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
146 Terms
Ionic Bonding
transfer of valence electron from metal donor and nonmetal recipient due to big differences in electronegativity, has electronegativity difference of 2 or higher
Chemical Bonds
lasting force of attraction between atoms, ions, or molecules that enable the formation of chemical compounds; they bond to achieve stability similar to the eight valence electrons of noble gases, following the octet rule
Valence Electrons
electrons on the outermost shell of an atom, can be identified based on the group of the element (except for helium having 2 electrons despite being a noble gas)
Recognizing Ionic Bonding
it is usually with a metal and nonmetal and the electronegativity difference is greater than 1.9; an electrostatic force of attraction between a cation and anion
Ionic Bond Properties
solid at room temp
high melting and boiling points
conduct electricity
soluble in water
Ion
atom that has gained a net charge
Cation
formed when atom loses electrons (positive charge)
Anion
formed when an atom gains electrons (negative charge)
Lewis Dot Symbol
used to keep track of valence electrons in the formation of chemical bonds, also used to display ionic and covalent bonds
Writing Formulas for Binary Ionic Bonds
Write the chemical symbols of both elements.
Determine the ionic charge of both elements, basing it on their groups in the periodic table.
Crisscross the ionic charges to be the subscripts so that the charge of one element is the subscript of another.
If possible, simplify the subscripts to the smallest ratio
Naming Ionic Compounds
Name the metal element.
Name the nonmetal element and add the suffix -ide to it
Covalent Bonding
sharing of valence electrons between two nonmetals due to close values of electronegativity
Covalent Bond Properties
exist as solid, liquid, and gas
low melting and boiling points
do not conduct electricity and heat
Recognizing Covalent Bonding
usually between two nonmetals and has an electronegativity difference of 0 to 1.9
Writing Formula of Binary Covalent Compounds
Write the chemical symbol based the arrangement of their names.
Determine the subscripts based on the given prefix
Writing Names of Binary Covalent Compounds
Write the name of the two nonmetals.
Add a prefix to both based on the subscripts.
Add the suffix -ide at the end of the 2nd nonmetal.
Writing Names of Binary Covalent Compounds with Transition Metals (Stock System)
Cation/Metal (Roman Numeral Representing Charge) + Nonmetal Anion
Writing Names of Binary Covalent Compounds with Transition Metals (Classical System)
For the cation with less charges, use “-ous,” for the cation with more charges use “-ic.” Use the latin name/origin name of the cations.
mono-
1 atom
di-
2 atoms
tri-
3 atoms
tetra
4 atoms
penta
5 atoms
hexa-
6 atoms
hepta-
7 atoms
octa-
8 atoms
nona-
9 atoms
deca-
10 atoms
Metallic Bond
attraction between metallic cation and the sea of delocalized electrons, only between metals
Metallic Bond Properties
malleable and ductile
conduct electricity
manifest hardness and strength
s-orbital
spherical shaped orbital, in energy levels 1-7
p-block
dumbell shaped orbital, in energy levels 2-7
d-block
clover leaf shaped, in energy levels 3-6
f-block
orbital with a complicated shape, in energy levels 4-5
The Latin name of Iron (Fe)
Ferrum
The Latin Name of Copper (Cu)
Cuprum
The Latin name of Iron (Fe)
Ferrum
The Latin name of Antimony (Sb)
Stibium
The Latin name of Gold (Au)
Aurum
The Latin name of Lead (Pb)
Plumbum
The Latin name of Mercury (Hg)
Hydragyrum/Mercury
The Latin name of Potassium (K)
Kalium
The Latin name of Silver (Ag)
Argentum
The Latin name of Sodium (Na)
Natrium
The Latin name of Tin (Sn)
Stannum
The Latin name of Tungsten (W)
Wolfram
Ternary Compound
a compound with three or more atoms, usually has a metallic cation and a polytatomic anion
Naming Ternary Compound
For compounds with more than three elements, combine the names of the metallic cation then the polyatomic anion
Valence Shell Electron Pair Repulsion (VSEPR) Theory
electrons surrounding atoms exert repulsive forces against each other, which means a molecule will take a shape where this will be minimized
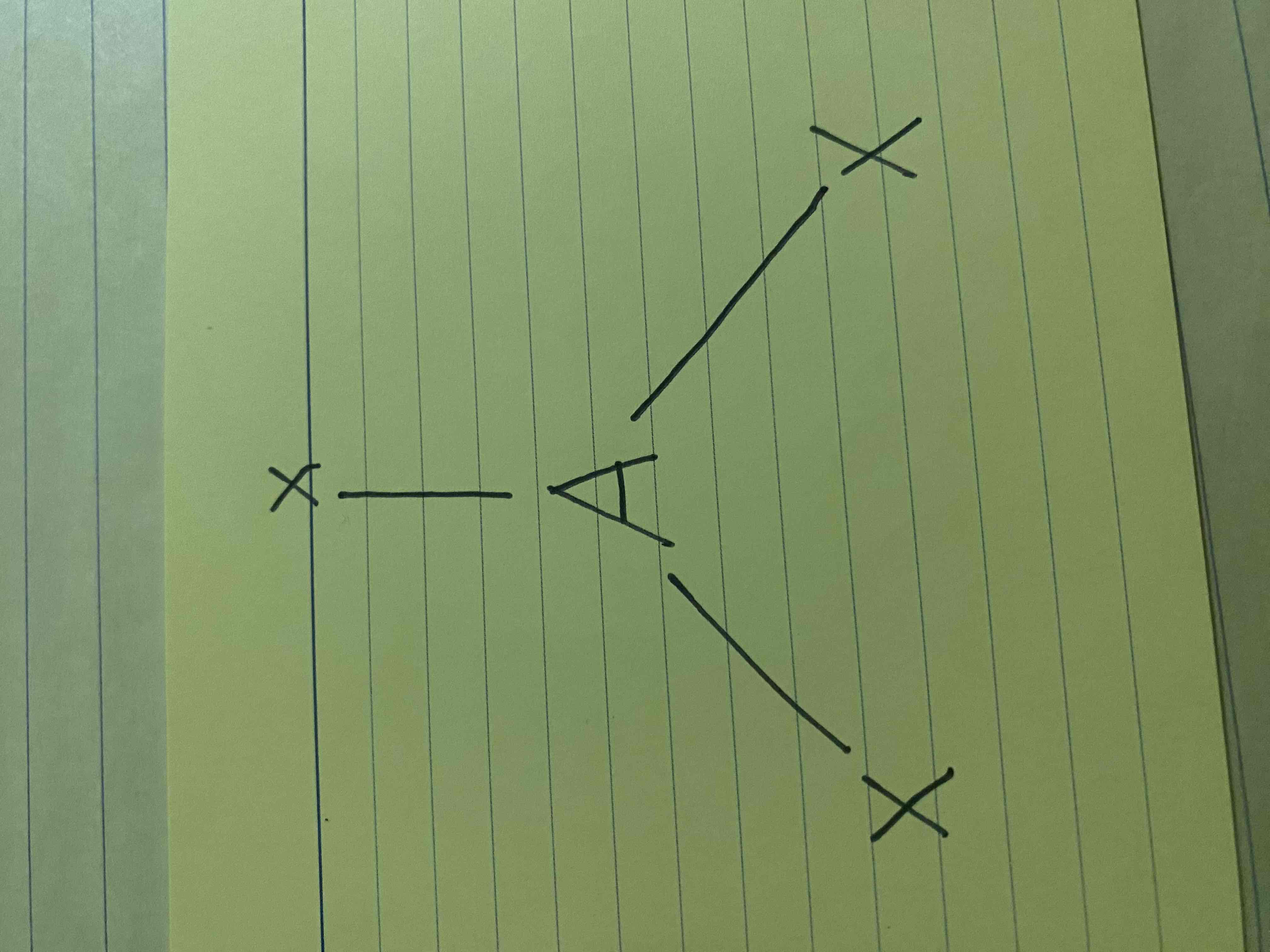
Central Atom
Represented by A, it is where all the bonds are attached to.
Electron Groups
consists of bonding groups and lone pairs
Bonding Groups
Represented by X, these are the bonds formed between the central atom and the other atoms
Lone Pairs
Represented by E, these are pairs of electrons that did not form a bond
Linear Electron Geometry
2 electron groups; sp hybridization; 180º
Trigonal Planar Electron Geometry
3 electron groups; sp² hybridization; 120º
Tetrahedral Electron Geometry
4 electron groups; sp³ hybridization; 109.5º
Trigonal Bipyramidal Electron Geometry
5 electron groups; sp³d hybridization and 120º (in plane) and 90º (above and below plane) for all VSPER classes except linear which has 180º
Octahedral Electron Geometry
6 electron groups; sp³d² hybridization and 90º
Linear Molecular Geometry
AX2 (2 bonds, 0 lone pairs)
AX2E3 (2 bonds, 3 lone pairs)
AX2E4 (2 bonds, 4 lone pairs)
Trigonal Planar Molecular Geometry
AX3 (3 bonds, 0 lone pairs)
Bent Molecular Geometry
AX2E (2 bonds, 1 lone pair)
AX2E2 (2 bonds, 2 lone pairs)
Tetrahedral Molecular Geometry
AX4 (4 bonds, 0 lone pairs)
Trigonal Pyramidal Molecular Geometry
AX3E (3 bonds, 1 lone pair)
Trigonal Bipyramidal Molecular Geometry | Composition
AX5 (5 bonds, 0 lone pair)
Seesaw Molecular Geometry | Composition
AX4E (4 bonds, 1 lone pair)
T-Shaped Molecular Geometry | Composition
AX3E2 (3 bonds, 2 lone pairs)
AX3E3 (3 bonds, 3 lone pairs)
Octohedral Molecular Geometry | Composition
AX6 (6 bonds, 0 lone pairs)
Square Pyramidal Molecular Geometry | Composition
AX5E (5 bonds, 1 lone pair)
Square Planar Molecular Geometry | Composition
AX4E2 (4 bonds, 2 lone pairs)
Predicting Molecular Geometry
Draw the Lewis Structure of the molecule
Count the number of bonds and lone pairs based on the central atom. Write these as the AXE notation.
Determine the shape based on the given notation
Draw the molecule based on the shape.
What is the line if the bond is on the plane of the paper?
Straight line
What is the line if the bond is coming out of the page towards us?
Triangle
What is the line if the bond is going into the page away from us?
Broken line
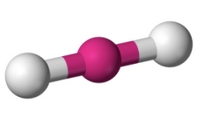
What molecular geometry is shown here?
Linear
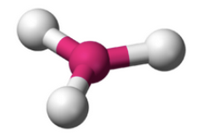
What molecular geometry is shown here?
Trigonal Planar
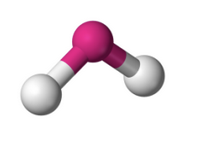
What molecular geometry is shown here?
Bent/Angular
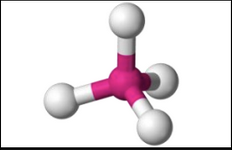
What molecular geometry is shown here?
Tetrahedral
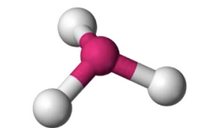
What molecular geometry is shown here?
Trigonal Pyramidal
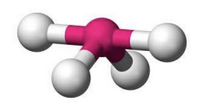
What molecular geometry is shown here?
Trigonal Bipyramidal
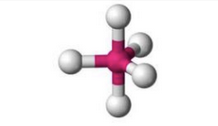
What molecular geometry is shown here?
Seesaw
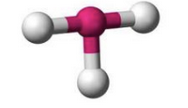
What molecular geometry is shown here?
T-Shape
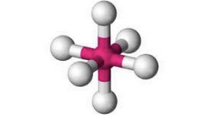
What molecular geometry is shown here?
Octahedral
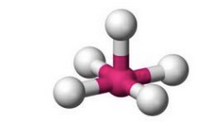
What molecular geometry is shown here?
Square Pyramidal
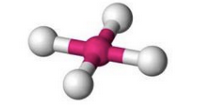
What molecular geometry is shown here?
Square Planar

What molecular geometry is shown here?
Linear (AX2)
What molecular geometry is shown here?
Trigonal Planar
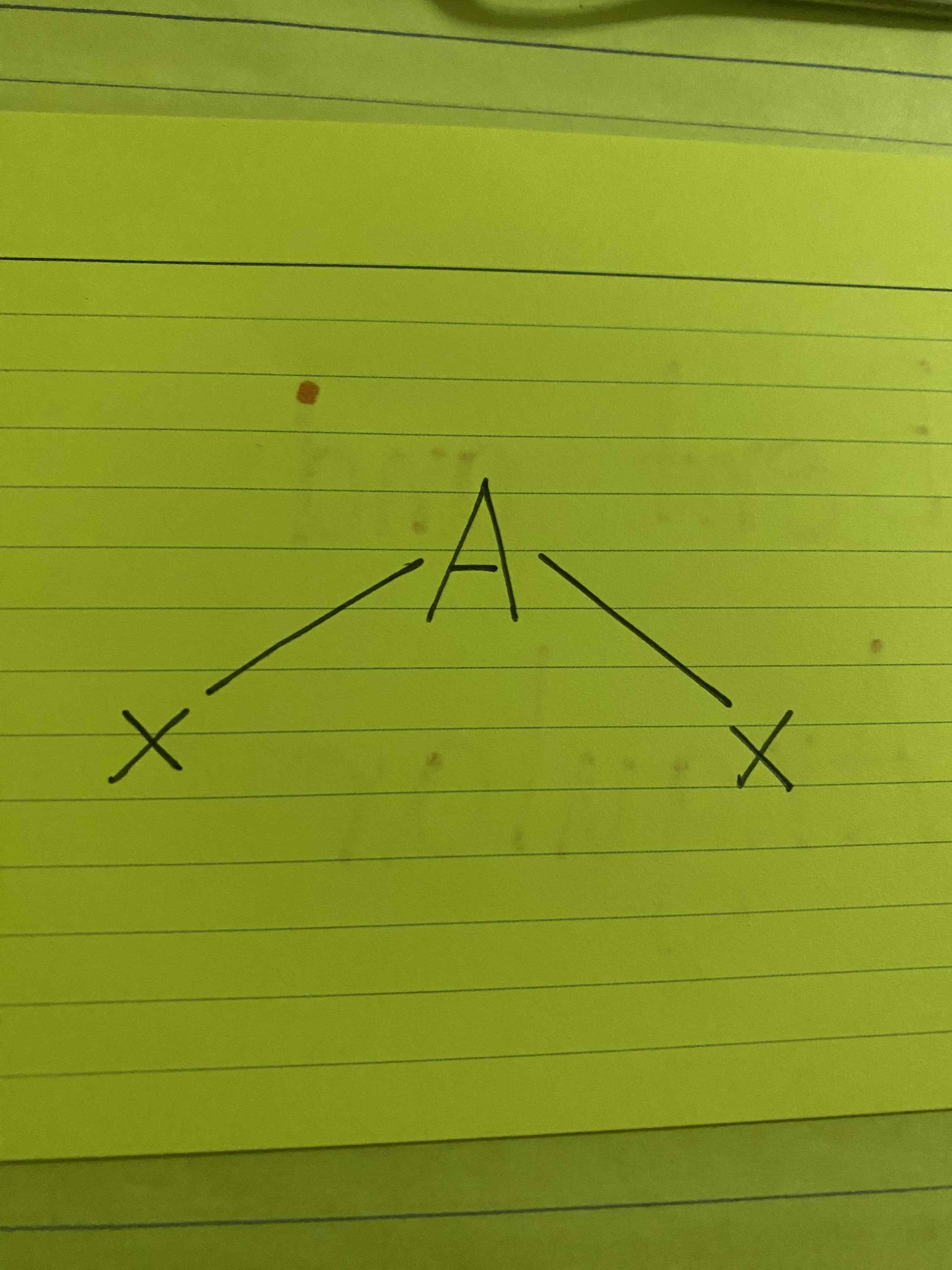
What molecular geometry is shown here?
Bent
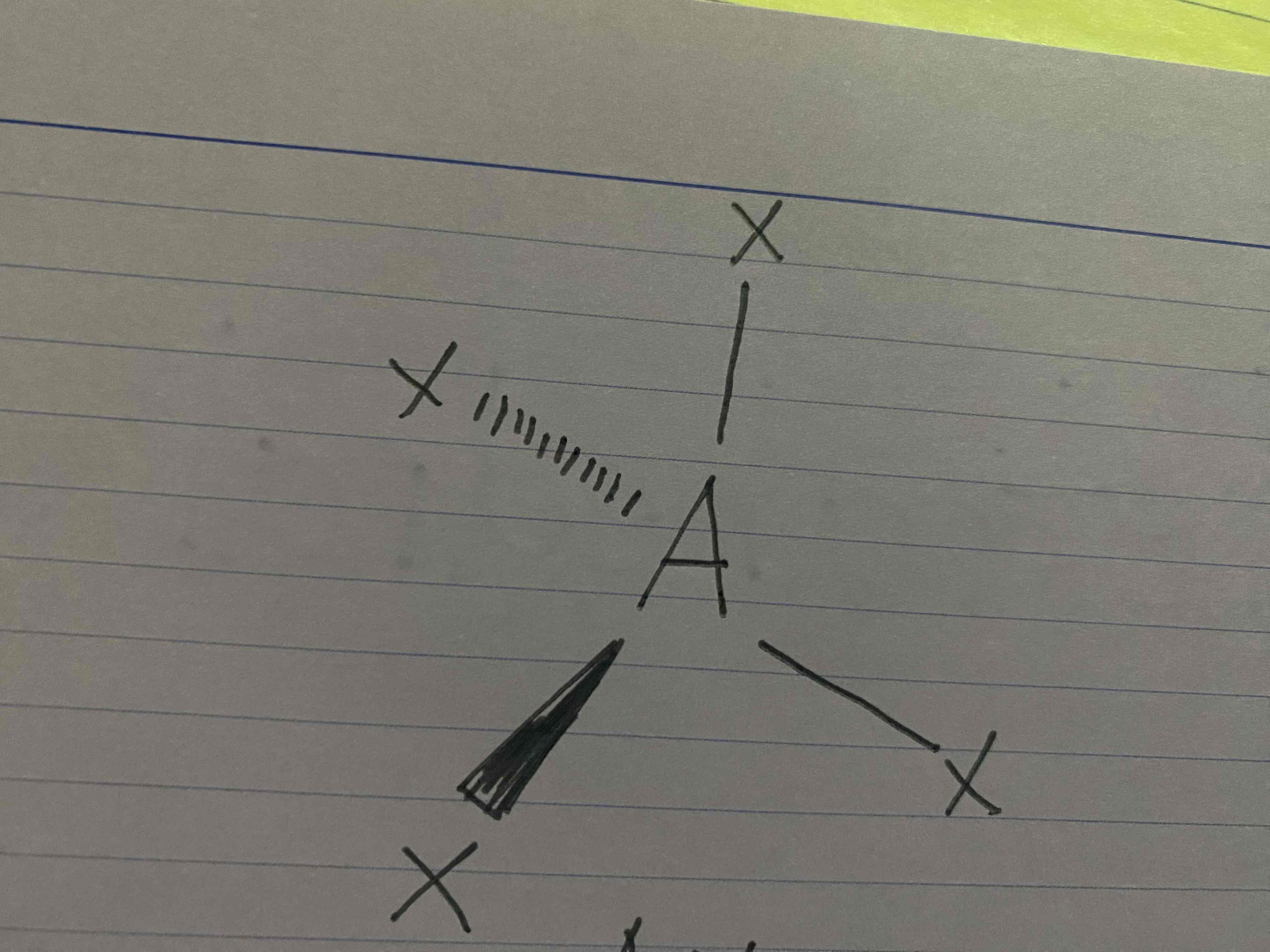
What molecular geometry is shown here?
Tetrahedral
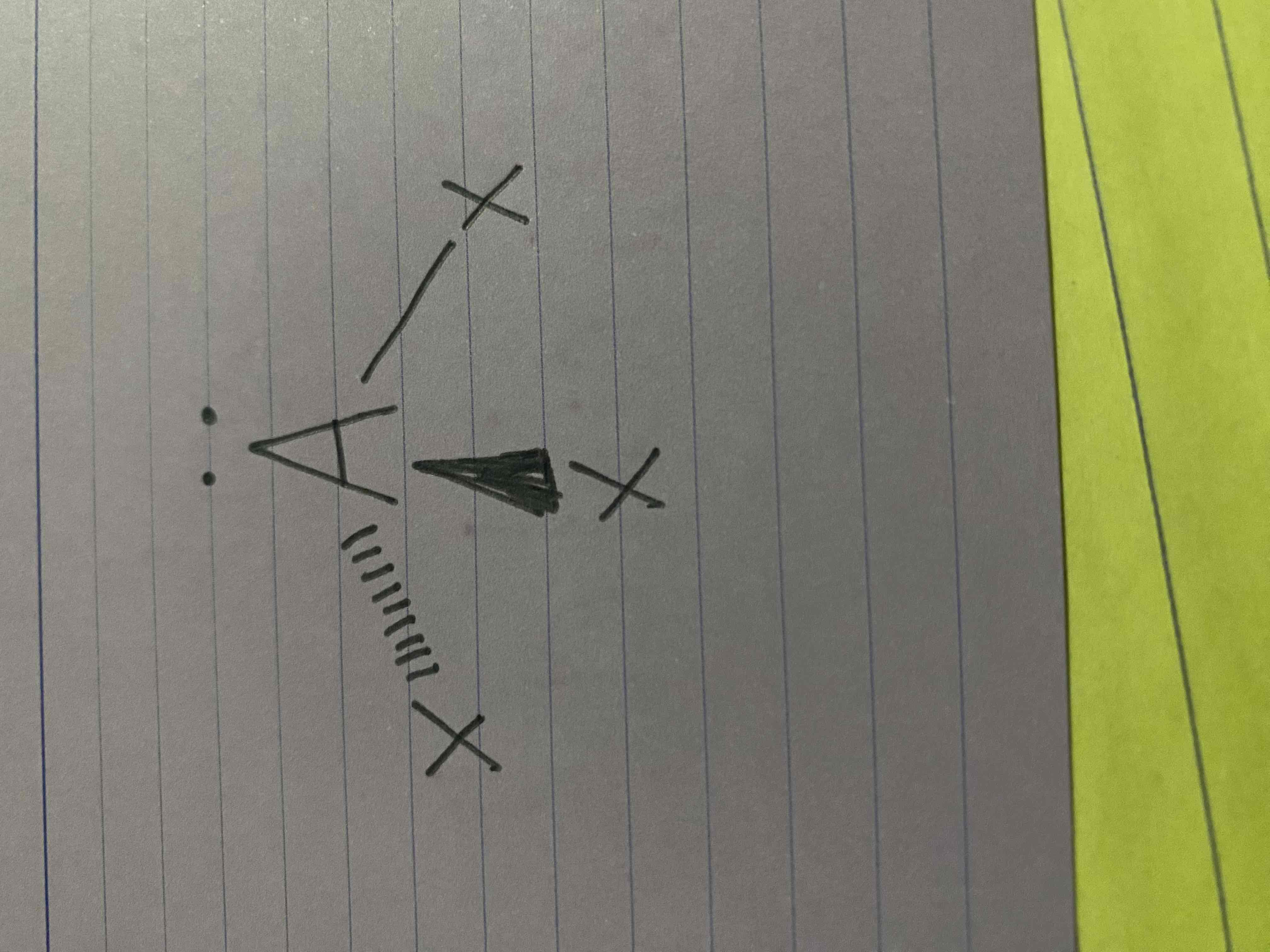
What molecular geometry is shown here?
Trigonal Pyramidal
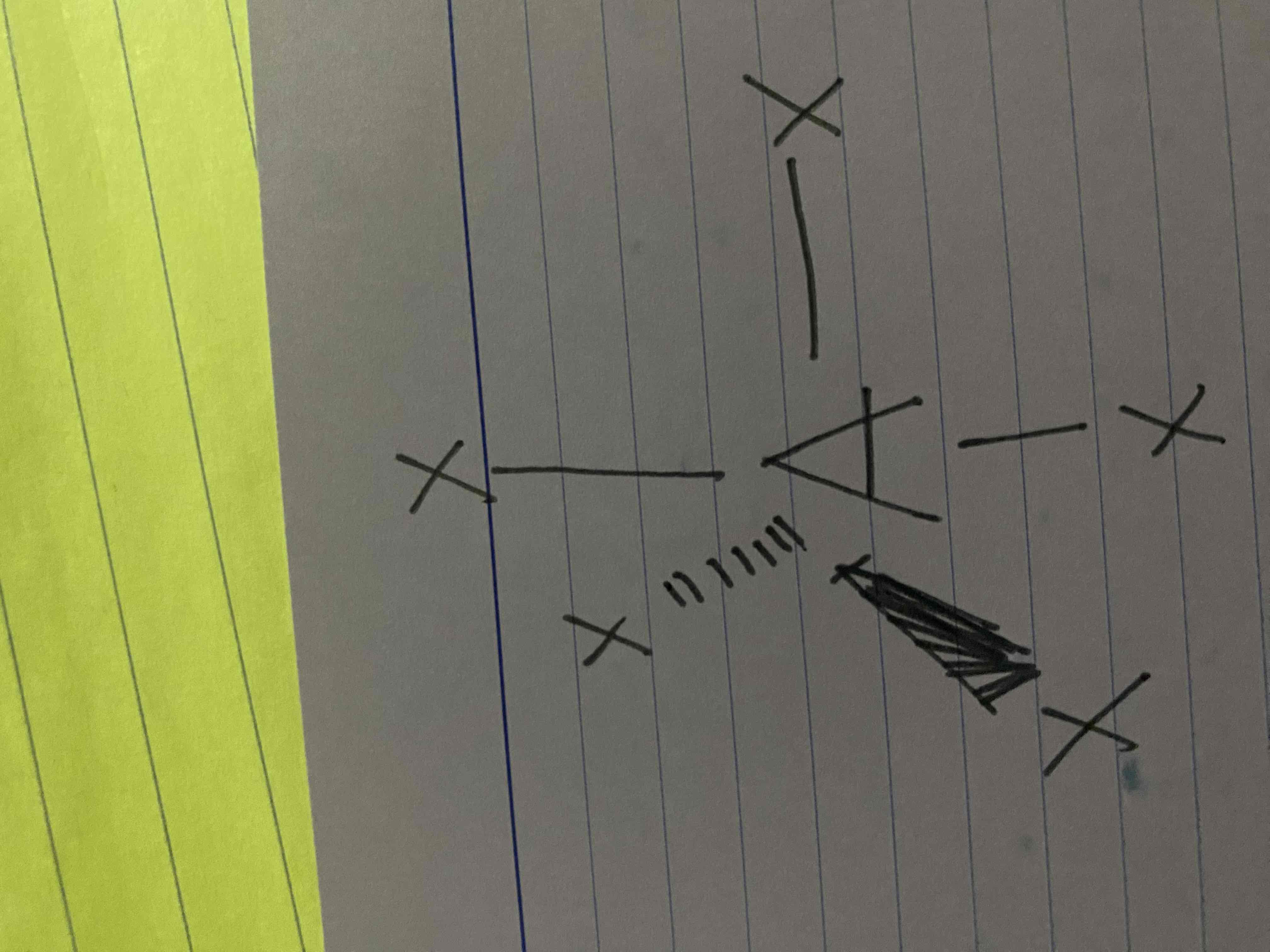
What molecular geometry is shown here?
Trigonal Bipyramidal
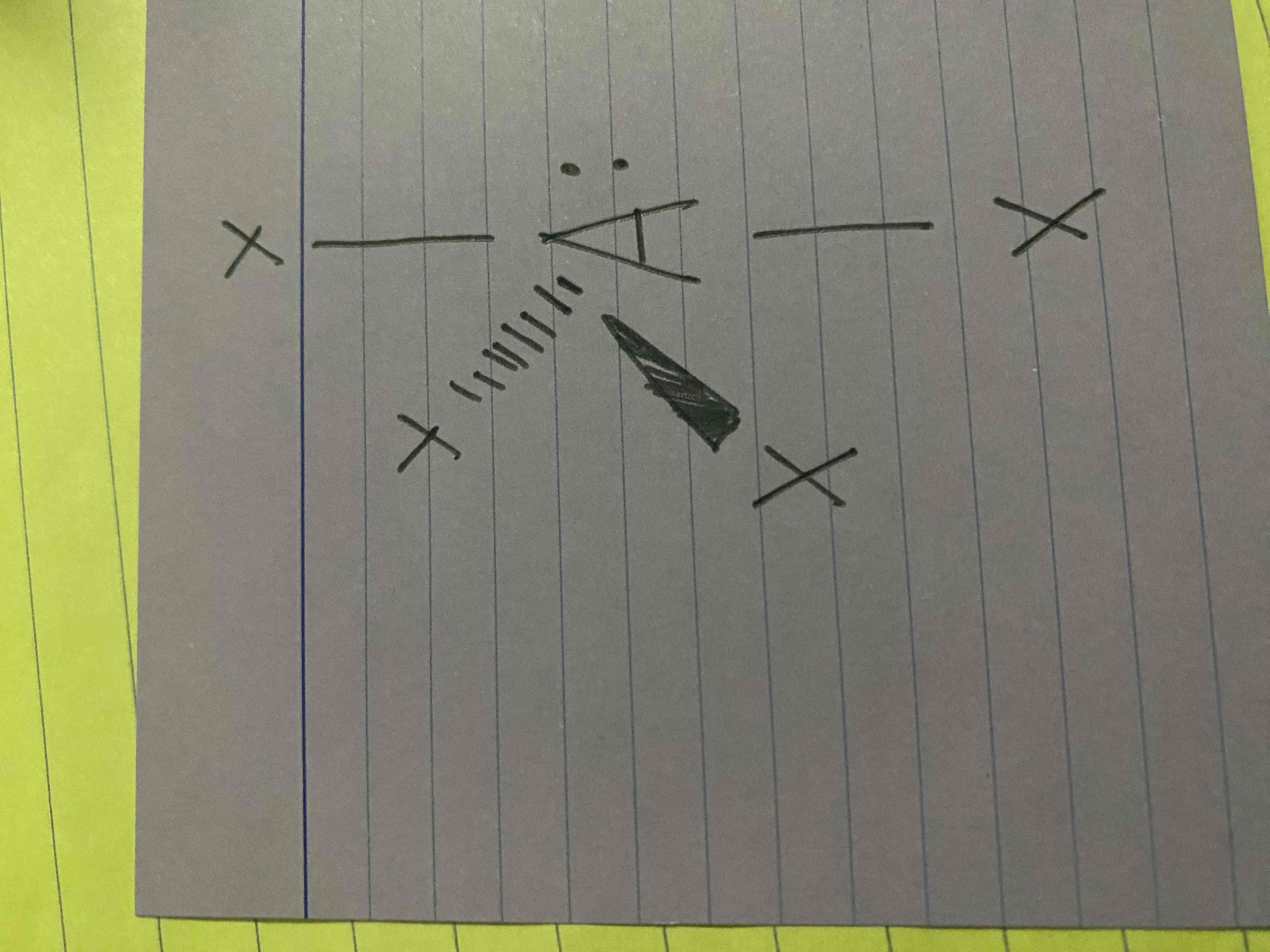
What molecular geometry is shown here?
Seesaw
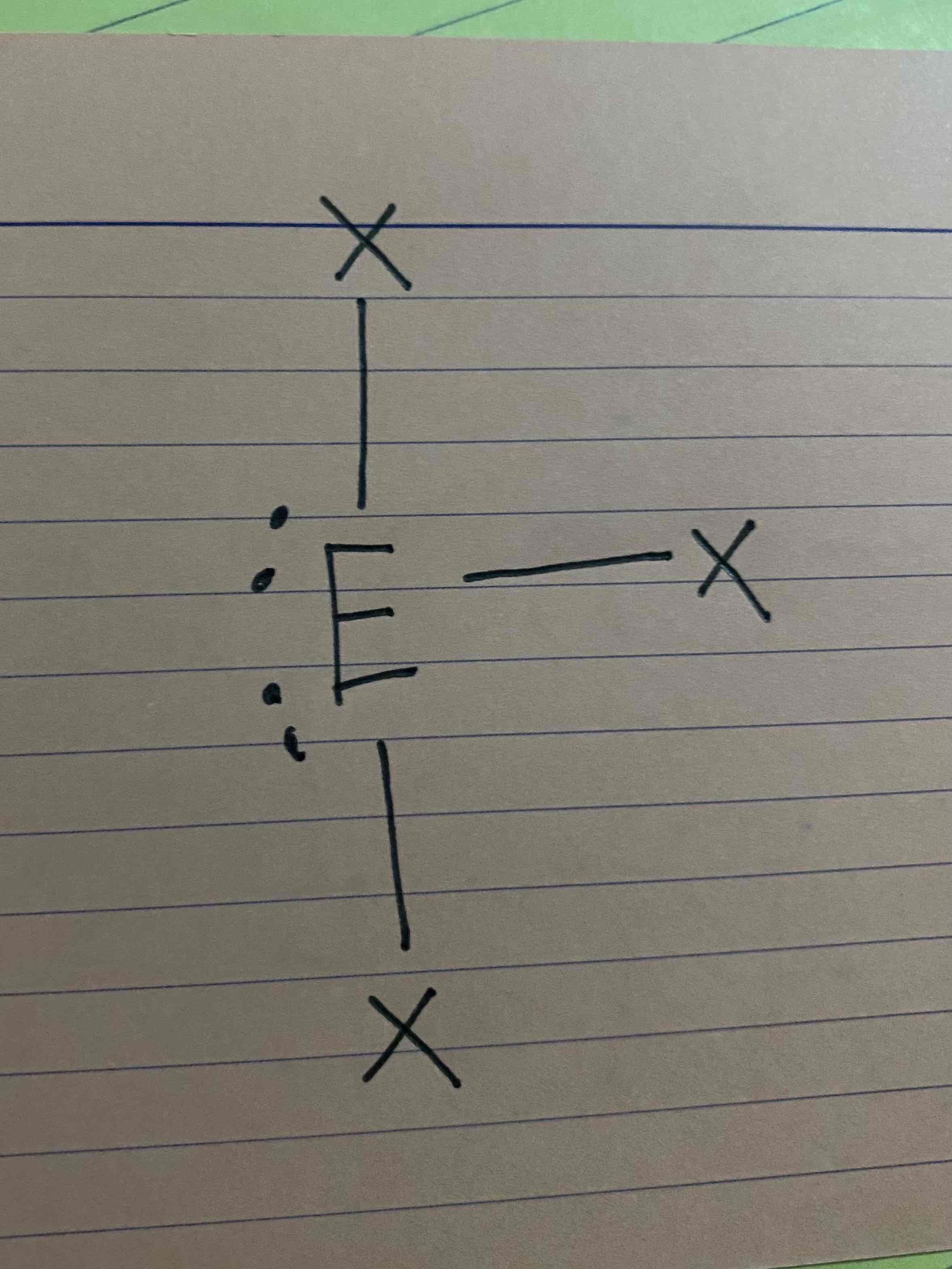
What molecular geometry is shown here?
T-shape (AX3E2)
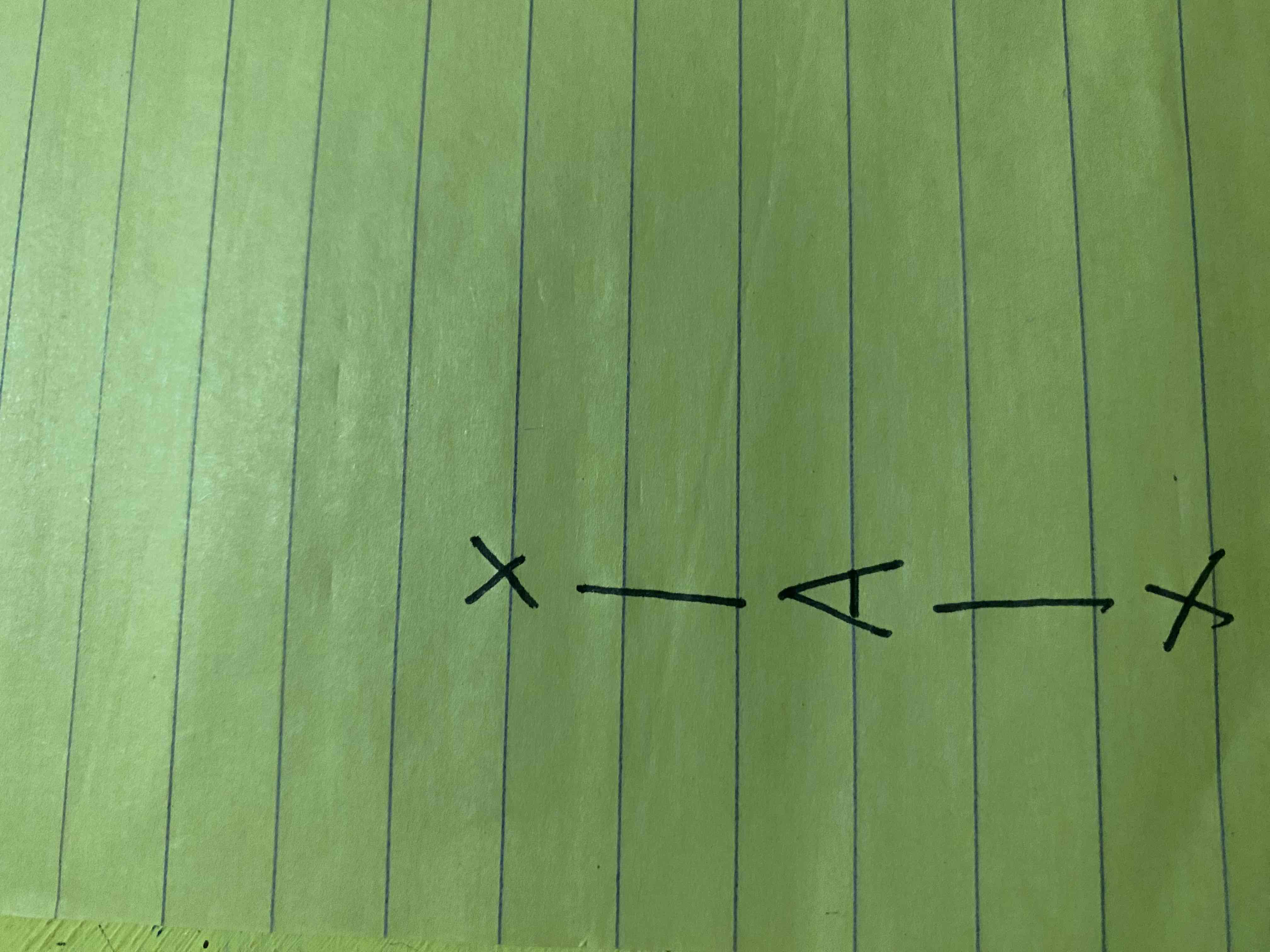
What molecular geometry is shown here?
Linear (AX2E3 and AX2E4)
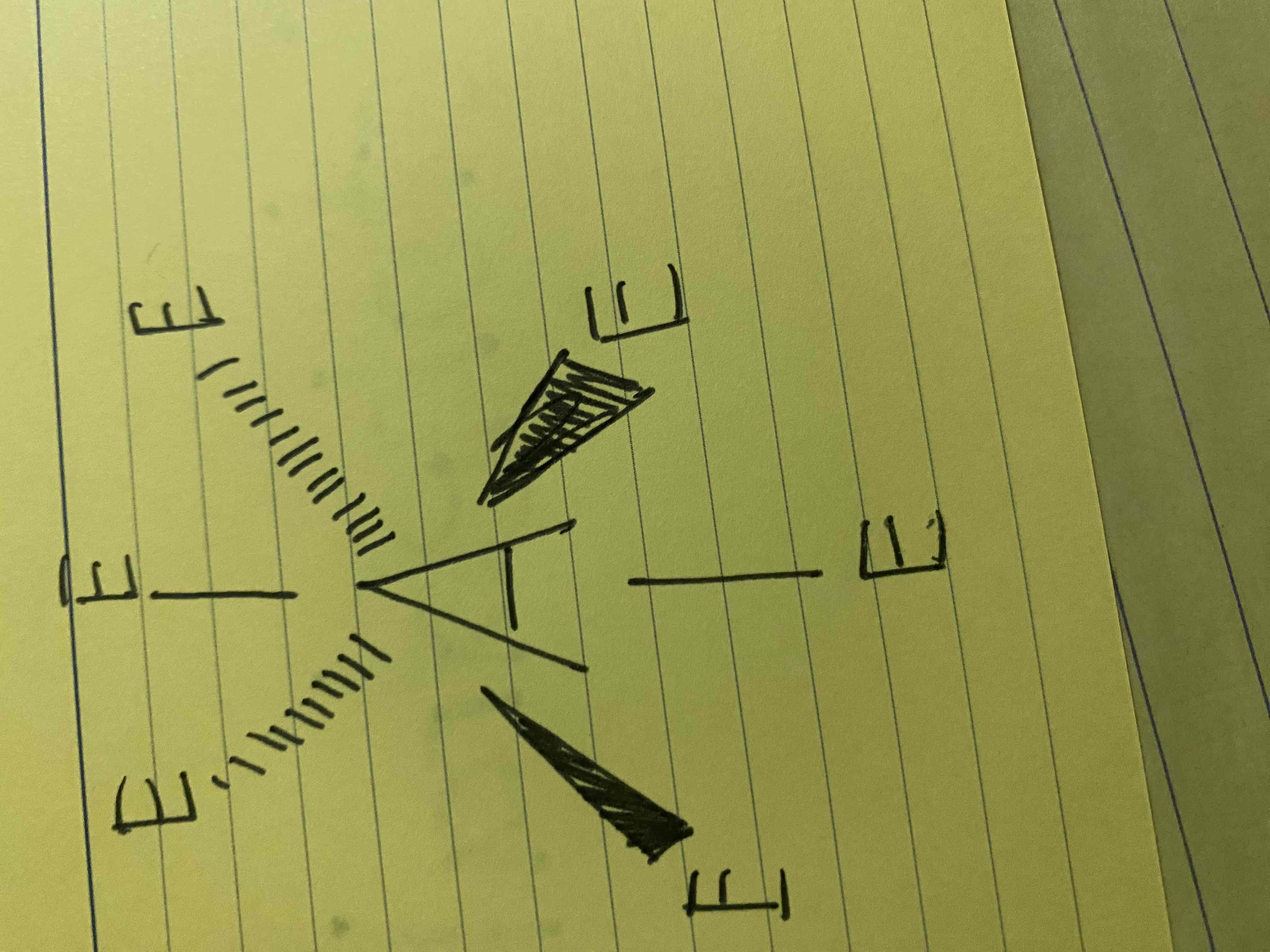
What molecular geometry is shown here?
Octahedral
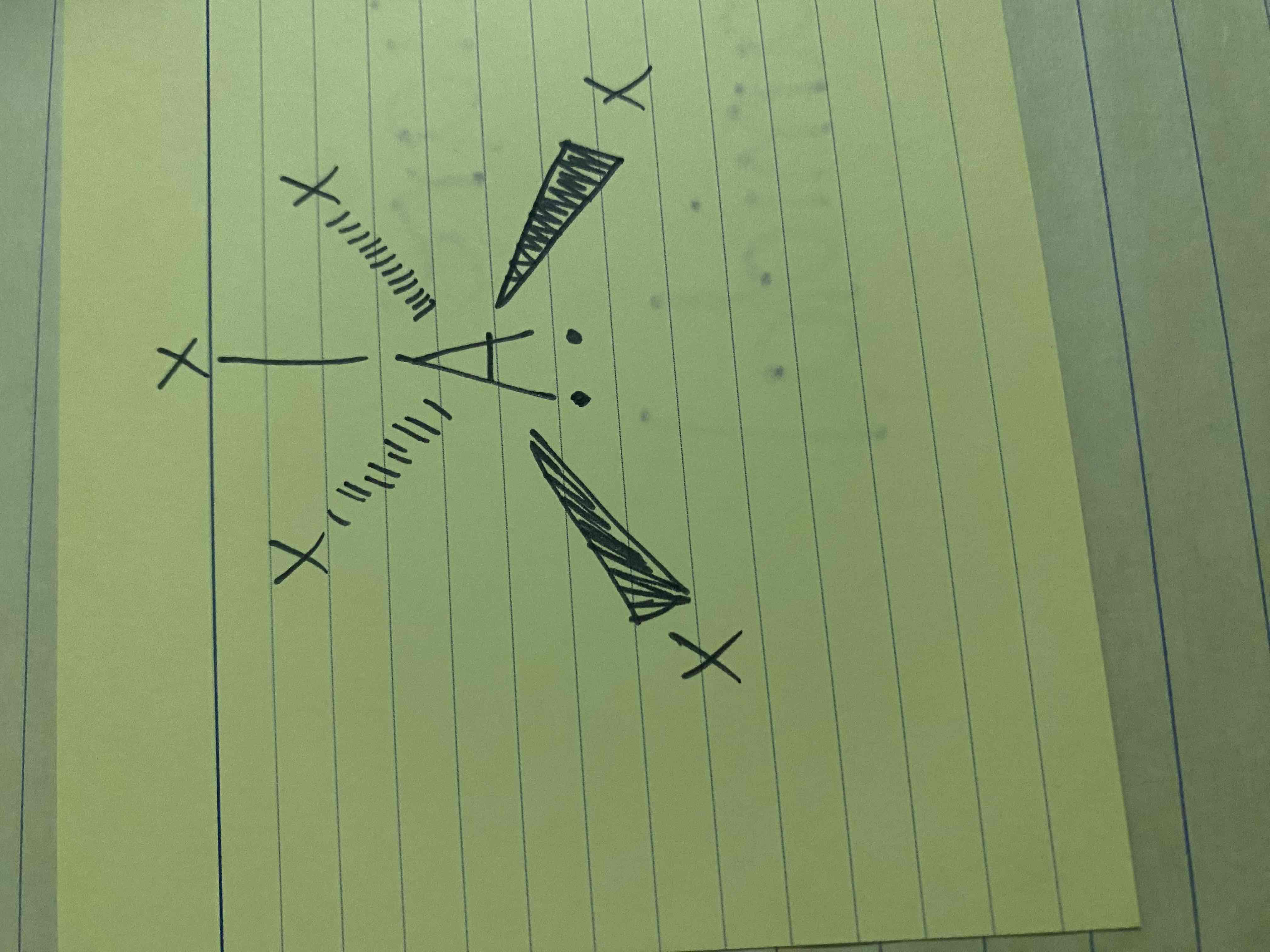
What molecular geometry is shown here?
Square Pyramidal
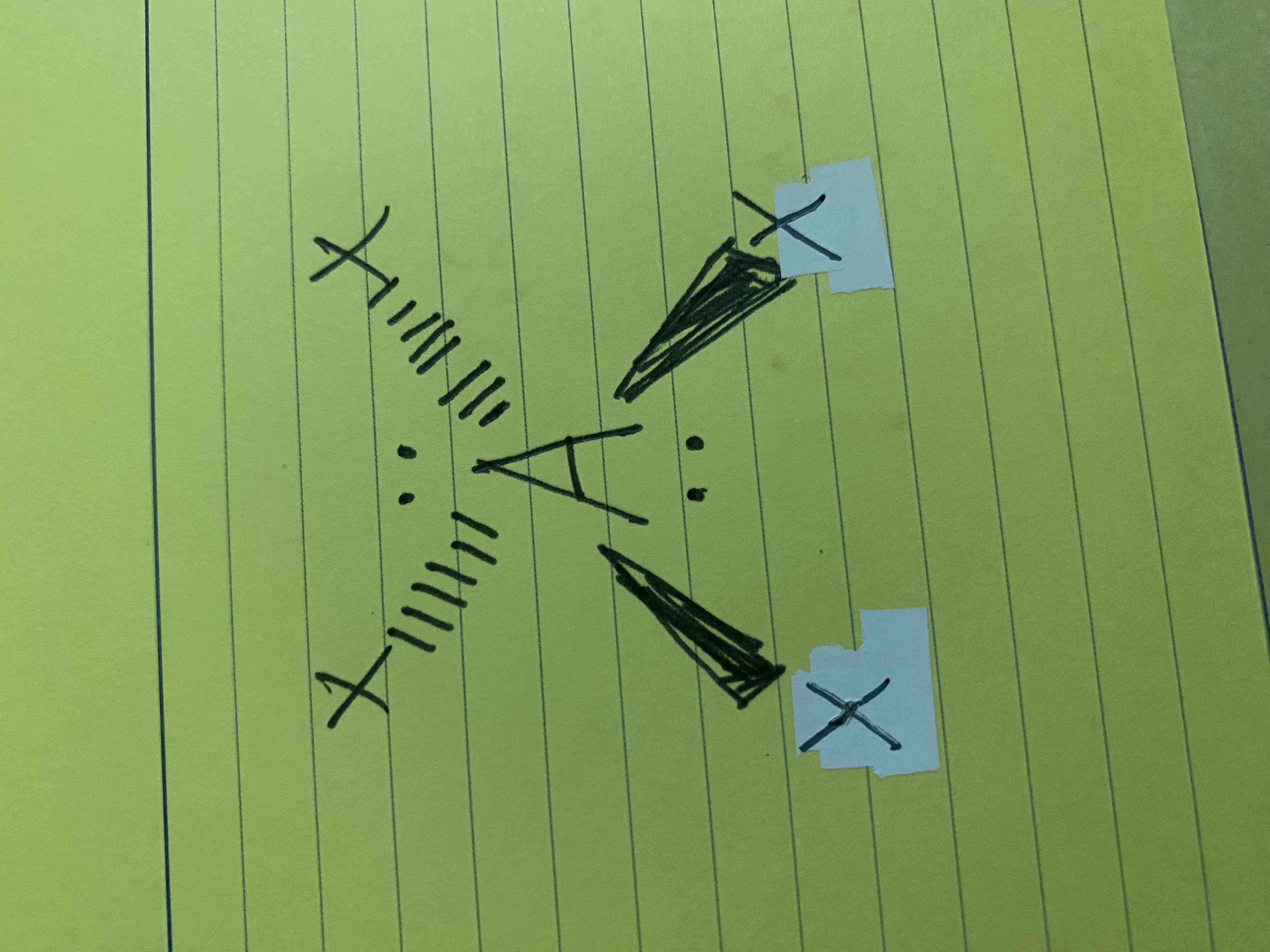
What molecular geometry is shown here?
Square Planar
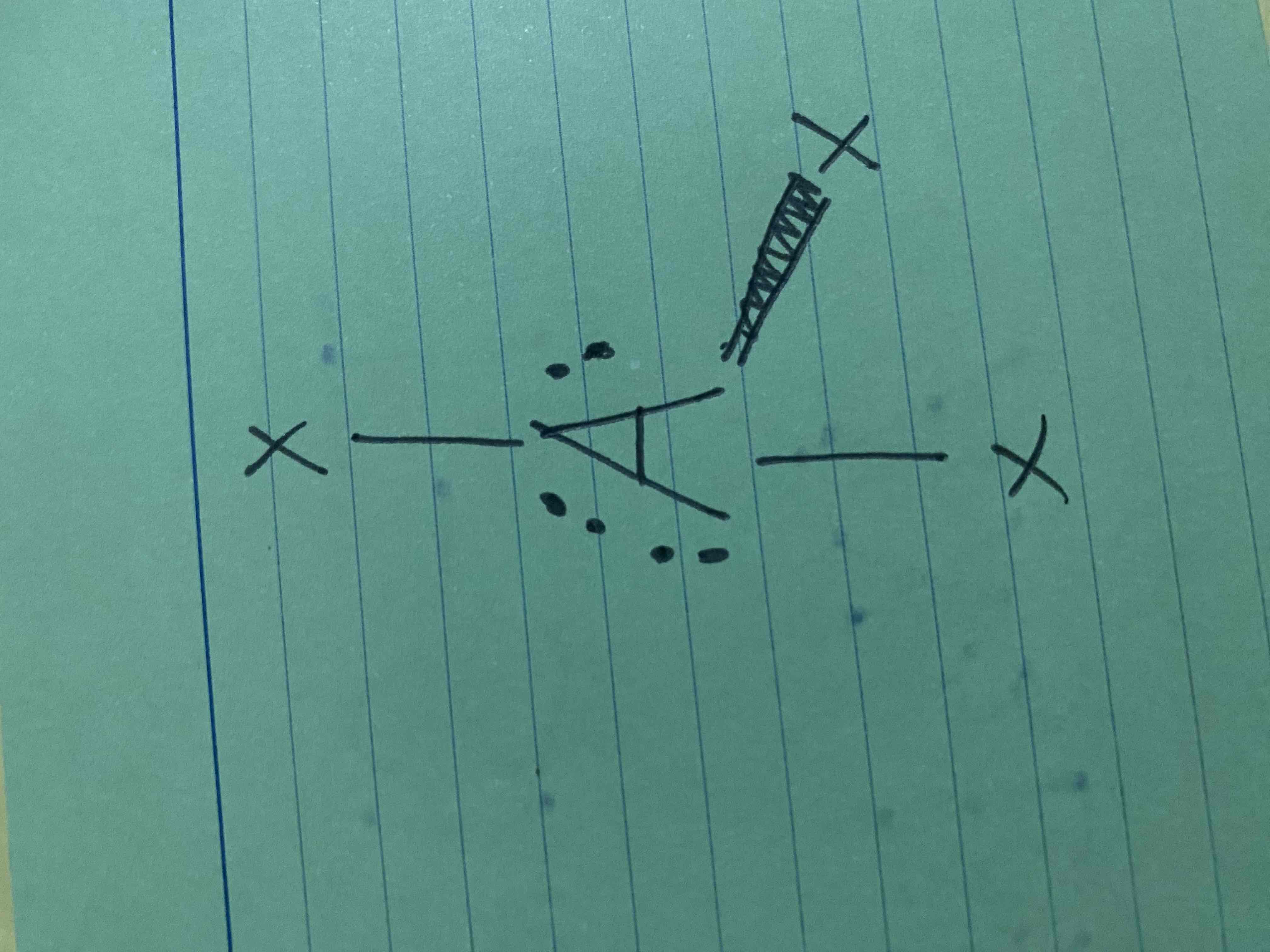
What molecular geometry is shown here?
T-shape (AX3E3)
Electronegativity
ability or tendency of an atom to attract electrons and thus form bonds.
Electronegativity Trends
an element’s electronegativity increases from left to right and bottom to top of the periodic table
Nonpolar Covalent Bond
there is equal sharing of electrons between atoms; electronegativity difference is less than 0.5