3.2.2. Group 2: The Alkaline Earth Metals
1/44
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
45 Terms
Group 2 Elements: the alkaline earth metals
Trend down a group in atomic radius: Increases
Down the group electrons are added to new shells which are further from the nucleus and the electron shielding increases.
Therefore there is less electrostatic force of attraction between the nucleus and the outer electron.
Trend down a group in 1st ionisation energy: Decreases
Down the group electrons are added to new shells which are further from the nucleus and the electron shielding increases. (Therefore there is less electrostatic force of attraction between the nucleus and the outer electron).
So less energy is required to remove the outer electron.
Trend down a group in melting and boiling point: Decreases
All group 2 elements have a giant metallic lattice structure.
Down the group: Ionic radius increases, (outer electron further from the nucleus and more shielding).
Electrostatic attraction between metal ions and delocalised electrons gets weaker (strength of metallic bonds decreases).
So less energy is required to overcome the metallic bonding.
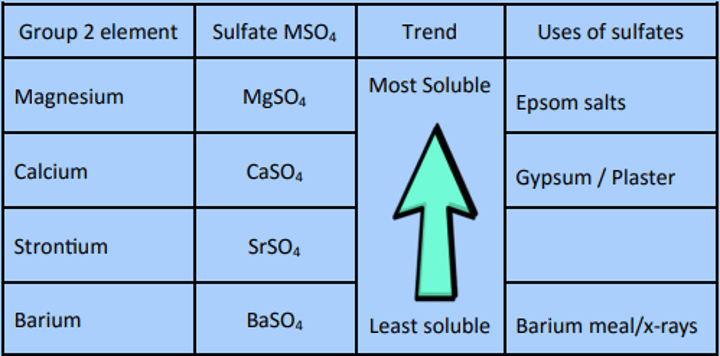
Sulfates
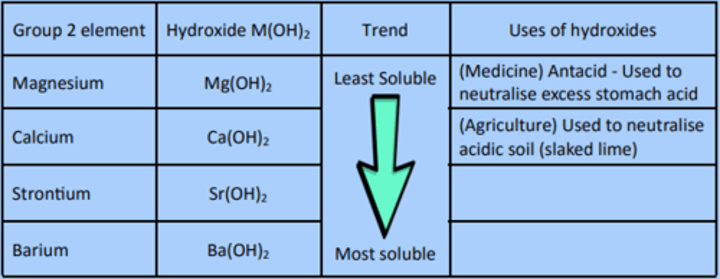
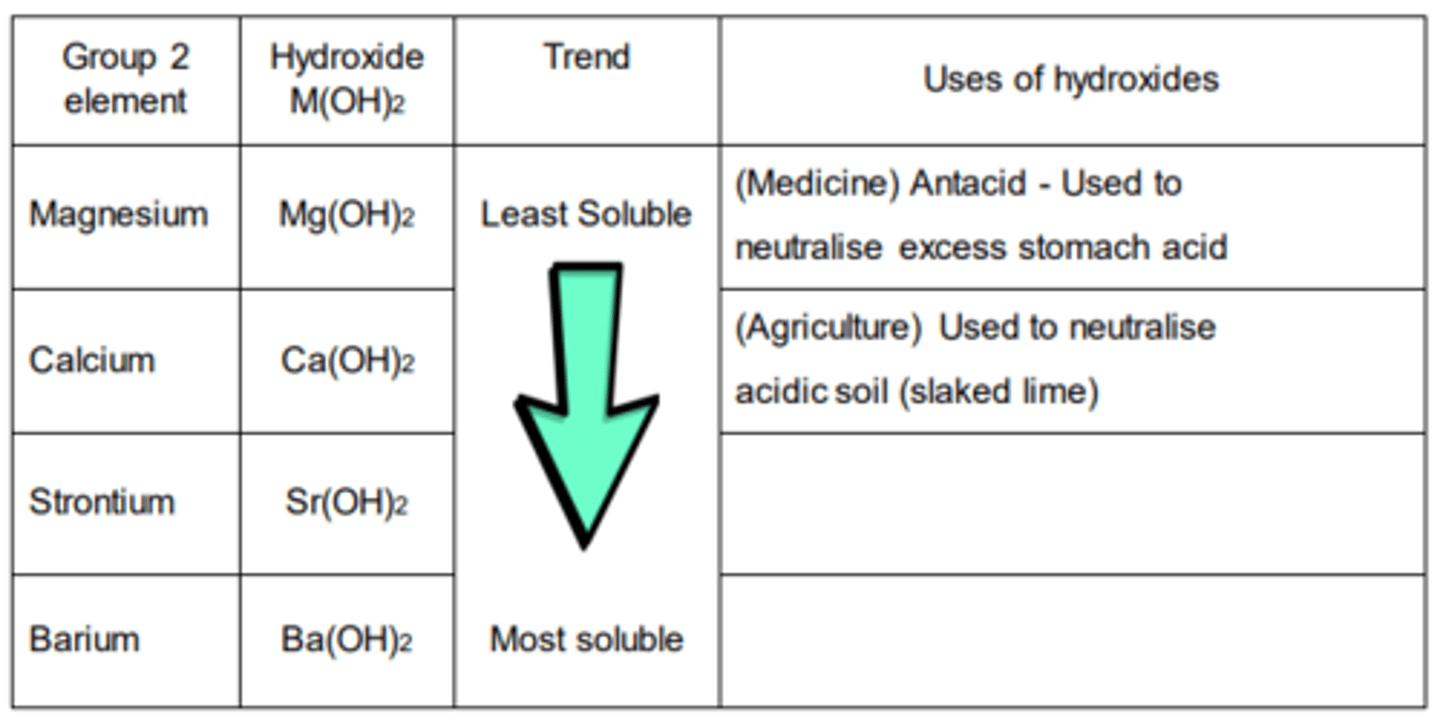
Hydroxides
Be
Does not react with water
Mg
Magnesium reacts very slowly with water (H2O (l) ) but reacts more quickly with steam (H2O (g)).
Equation with water:
Mg (s) + 2H2O (l) → Mg(OH)2 (s) + H2 (g)
Slight white ppt.
Equation with steam:
Mg (s) + H2O (g) → MgO (s) + H2 (g)
White solid
Ca
Calcium reacts steadily with cold water. The solution goes cloudy as the hydroxide is not very soluble and forms a slight white ppt.
Equation:
Ca (s) + 2H2O (l) → Ca(OH)2 (s) + H2 (g)
Slight white ppt.
Sr
Strontium reacts vigorously with cold water forming a colourless solution.
Equation:
Sr (s) + 2H2O (l) → Sr(OH)2 (aq) + H2 (g)
Ba
Barium reacts vigorously with cold water forming a colourless solution.
Equation:
Ba (s) + 2H2O (l) → Ba(OH)2 (aq) + H2 (g)
Flame Tests for group 2 metal ions:
Clean nichrome wire with HCl, dip into group 2 ionic solid, heat in blue Bunsen flame.
Observations:
Ca2+ = brick red
Sr2+ = red
Ba2+ = pale green
Test for sulfate ions
Reagent = barium chloride solution (i.e. a solution of barium ions)
1) Acidify test solution with hydrochloric acid -removes impurities.
2) Add barium chloride solution.
3) If sulfate ions are present a white precipitate of barium sulfate will be seen - as it is insoluble in water.
Simplest ionic equation:
Ba2+(aq) + SO42−(aq) → BaSO4(s)
Use of magnesium chloride in the extraction of titanium: Kroll process
Step 1
TiO2 (solid) is converted to TiCl4 (liquid) at 900 °C: TiO2 + 2Cl2 + 2C → TiCl4 + 2CO
Step 2
Magnesium (or sodium) is used as a reducing agent:
TiCl4 + 2Mg → Ti + 2MgCl2
The reaction is done in an inert argon (Ar) atmosphere which prevents the magnesium reacting with oxygen (from the air).
These reactions are exothermic which helps maintain the very high temperature, 900 °C.
Use of calcium salts in flue-gas desulfurisation:
To prevent SO2 from being released into the atmosphere when burning oil or coal (fossil fuels) in power stations using flue-gas desulfurisation.
Uses alkaline substances to remove the acidic sulfur dioxide eg. calcium oxide (CaO) from limestone (CaCO3):
CaO + SO2 → CaSO3
Definition of atomic radius:
The distance from the nucleus to the outermost
occupied electron energy level/shell.
State and explain the trend in atomic radius down group 2.
Atomic radius increases down the group;
Down the group electrons are added to new shells which are further from the nucleus and the electron shielding increases.
(Therefore there is less electrostatic force of attraction between the nucleus and the outer electron.)
Definition of first ionisation energy:
The enthalpy change when one mole of electrons is removed from one mole of gaseous atoms to form one mole of gaseous unipositive ions.
State and explain the trend in first ionisation energy for group 2 elements.
First ionisation energy decreases down the group;
Down the group, atomic radius increases (outer electron further from nucleus), and electron shielding increases.
So less energy is required to remove the outer electron.
(Smaller electrostatic force of attraction between nucleus and outer electron).
State and explain the trend in melting and boiling points for group 2 elements.
Melting and boiling points decrease down the group.
Down the group all have metallic structure, ionic radius increases, (charge density decreases) electrostatic attraction between metal ions and delocalised electrons gets weaker (strength of metallic bonds decreases), less energy required to overcome metallic bonding.
Reactions with water
Group 2 metals react with water to form metal hydroxides and hydrogen gas.
M (s) + 2H2O (l) → M(OH)2 (aq) + H2 (g)
During the reaction the Group 2 element is oxidised (loses two electrons) to form a 2+ ion.
The trend is for increasing reactivity with water down Group 2.
Beryllium Be
Does not react with water
Magnesium Mg
Magnesium reacts very slowly with water (H2O (l) ) but reacts more quickly with steam (H2O (g)).
Equations
With water:
Mg (s) + 2H2O (l) -> Mg(OH)2 (s) + H2(g)
Slight white ppt.
With steam:
Mg (s) + H2O (g) -> MgO (s) + H2 (g)
White solid
Calcium Ca
Calcium reacts steadily with cold water. The solution goes cloudy as the hydroxide is not very soluble and forms a slight white ppt.
Equation: Ca (s) + 2H2O (l) -> Ca(OH)2 (s) + H2 (g)
Strontium Sr
Strontium reacts vigorously with cold water forming a colourless solution.
Equation: Sr (s) + 2H2O (l) -> Sr(OH)2 (aq) + H2 (g)
Barium Ba
Barium reacts vigorously with cold water forming a colourless solution.
Equation: Ba (s) + 2H2O (l) -> Ba(OH)2 (aq) + H2 (g)
Write an equation for the reaction of Radium (Ra) with water.
Ra (s) + 2H2O (g) -> Ra(OH)2 (aq) + H2 (g)
Predict the reactivity of radium with water compared to the other Group 2 elements.
More reactive. Reactivity increases down the group.
State and explain the trend in first ionisation energy down Group 2.
Decreases. Electron becomes easier to remove as it is further from nucleus and there is shielding by inner filled shells.
For which group 2 metal would you observe a slight precipitate when adding it to water?
Calcium. Ca(OH)2 is only slightly soluble in water. (Mg will not react with water but reacts with steam to form the oxide MgO).
Hydroxides M(OH)2
Sulfates MSO4
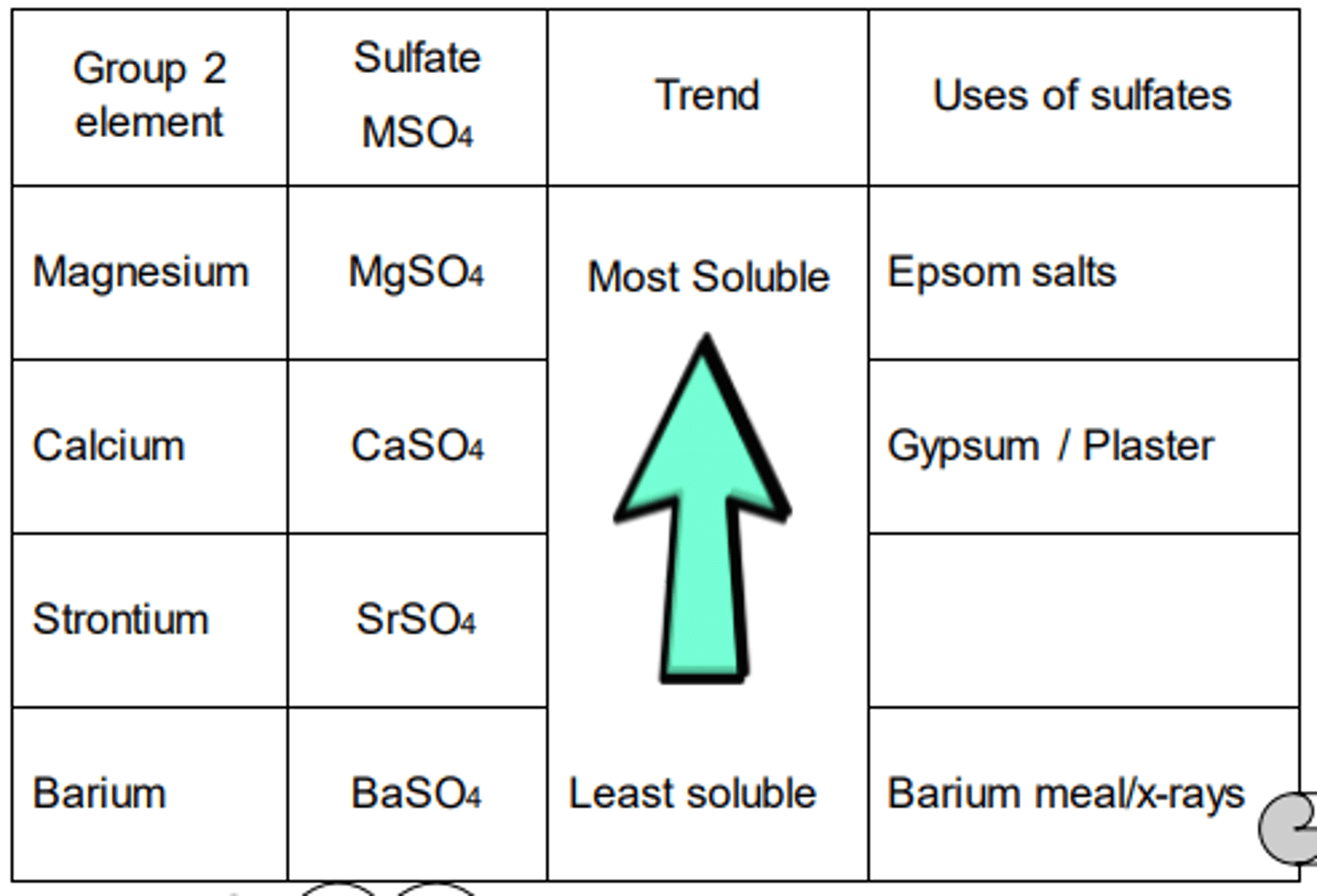
Remember that BaSO4 is insoluble. Then;
the other sulfates must be more soluble, and
the trend for the hydroxides is the opposite
Ba2+(aq) is toxic; so how can BaSO4 be used for a
barium meal?
Because barium sulfate is insoluble in water so
won't enter the blood stream.
(so the Ba2+(aq) ion is not formed.)
Use of magnesium in the extraction of titanium
The main titanium ore is rutile, containing TiO2.
Titanium cannot be extracted with carbon using a blast furnace because titanium carbide (TiC) is formed rather than titanium; TiC is very brittle.
Titanium is therefore extracted in two steps using the Kroll process. This is a batch process - it takes two separate steps and the materials used must be renewed each time.
Step 1
TiO2 (solid) is converted to TiCl4 (liquid) at 900 °C:
TiO2 + 2 Cl2 + 2 C -> TiCl4 + 2 CO
Step 2
Magnesium (or sodium) are used as a reducing agent to reduce Titanium(IV) chloride,TiCl4 to titanium, Ti.
TiCl4 + 2 Mg -> Ti + 2 MgCl2
(TiCl4 + 4 Na -> Ti + 4 NaCl)
The reaction is done in an inert argon (Ar) atmosphere which prevents the magnesium or
sodium reacting with oxygen (from the air).
These reactions are exothermic which helps to keep the reaction vessel at a very high
temperature, 900C.
Use of CaO or CaCO3 to remove SO2 from flue gases.
Fossil fuels such as petroleum, coal and natural gas contain sulfur. When the sulphur burns (reacts with oxygen) it produces sulfur dioxide which can then be released into the atmosphere to produce acid rain.
The two main examples of this are:
1) burning hydrocarbons which contain sulfur impurities in a car engine.
2) burning oil or coal (fossil fuels) in power stations.
1) Petrol engines will not work efficiently if sulphur is present in the fuel as it can block sensors. It is important that unleaded petrol is checked for sulfur contamination by fuel companies, and removed if present.
2) The release of SO2 is often prevented in factories and power stations using a process
called flue-gas desulphurisation.
1) Identify a chemical used in flue gas desulfurisation
CaO or CaCO3
2) Write the equation for the reaction that occurs during flue-gas desulfurisation.
CaO + SO2 -> CaSO3
3) State the type of reaction that is occurring.
Neutralisation / acid-base reaction
4) Give a use of a product of flue-gas desulfurisation
Plaster/gypsum
Important test for sulfate ions / test for barium ions
Reagent = barium chloride solution (i.e. a solution of barium ions)
1) Acidify test solution with hydrochloric acid - removes impurities.
2) Add barium chloride solution.
3) If sulfate ions are present a white precipitate of barium sulfate will be seen - as it is insoluble in water.
Explain why sulfuric acid cannot be used to acidify the solution in step one.
The barium ions Ba2+(aq) would react with the sulfate ions SO42-(aq) in the sulphuric acid to give a false positive result.
Ba2+(aq) + SO42-(aq) -> BaSO4(s)
Write an equation to show the production of the white precipitate.
Ba2+(aq) + SO42-(aq) -> BaSO4(s)
Write a brief method to test for barium ions.
1) Add sulfuric acid.
2) If barium ions are present a white precipitate of barium sulfate will be seen - as it is insoluble in water.
Important test for group 2 ions: Flame Tests
Method:
1. Dip the nichrome wire (a nickel-chromium alloy) into hydrochloric acid (to clean it).
2. Then dip the wire into the unknown compound (solid or solution).
3. Hold the wire in the pale blue Bunsen flame and observe the colour change in the flame.
Observations:
Group 2 metal Flame colour
Calcium, Ca2+ Brick red
Strontium, Sr2+ Red
Barium, Ba2+ Pale green