3.4 - Organic Analysis
1/15
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
16 Terms
Test Tube Reactions
Test Tube Reactions
What is the Positive result of a Bromine water test?
What gives the positive result
Orange → Colourless:
Alkenes
What is the Oxidising agent for Alcohols?
Potassium Dichromate (K2Cr2O7)
What is the Positive result of Potassium Dichromate:
Primary Alcohol
Secondary Alcohol
Tertiary Alcohol
Orange → Green:
Primary → Aldehyde → Carboxylic Acid
Secondary → Ketone
Tertiary → N/A
What solutions can you use to differentiate Aldehydes + Ketones?
Positive results → for both
Fehlings (Copper based solution) / Tollens (Silver mirror test) → Test for Aldehydes:
Fehlings = Blue → Red
Tollens = Silver mirror precipitate formed
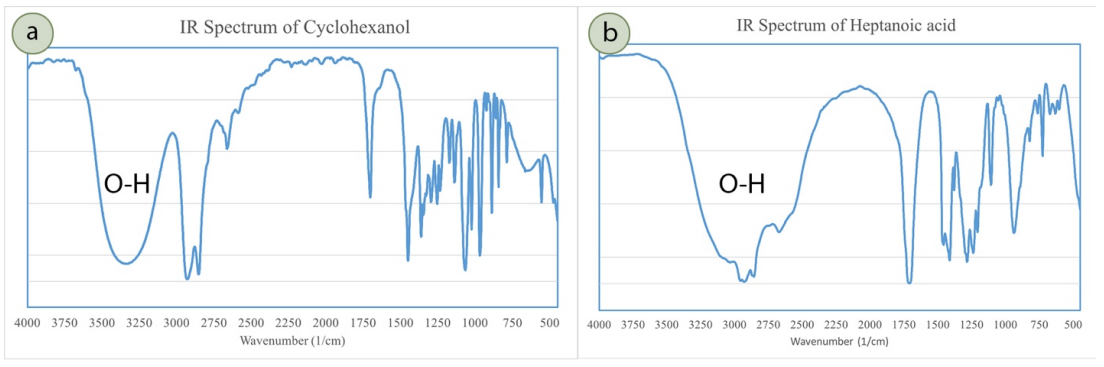
How do you differentiate between -OH in IR Spectroscopy:
Alcohol
C.Acid
Alcohol = Whale head → (more of a curve on the left of the spiky notch)
C.Acid = Iceberg → (large + wide notch)
Define the Fingerprint region
Region in an IR Spectrogram between 500cm-1-1500cm-1 unique to a compound → computers can only differentiate this by comparing the sample to known compounds + finding exact matches
How do IR Spectrograms create the Spectrographs?
Bonds in molecules have their own vibrational frequencies which increase as IR is absorbed, then released and detected to form the graph
What is the IR cycle of Global Warming? (4 steps)
IR is emitted from the sun to earth
In the night as the earth cools, IR is re-emitted back out into the atmosphere → some to space
Molecules like C=O from Carbon dioxide absorb this re-emitted IR
The molecules then re-emit the IR to surrounding molecules which absorb + emit IR to the earth → having an overall net increase in Global Temperature
What 6 types of Molecules absorb IR in the atmosphere?
CO2
H2O
NOx
CH4
O3
CFC’s
Additional Mass Spec
Additional Mass Spec
Why are HRMs used over LRMs?
HRMs have a higher Mr resolution of a higher degree → LRMs have whole number resolution so can give the same Mr for different compounds
When are HMRs useless?
Isomers with same Mr
How do we determine isomers from eachother?
Which type of Mass Spec
Look at fragment peaks to differentiate samples, small number of whole isomer molecules are detected
Electron Impact
Why are the fragment peaks larger than molecule peaks?
As the Mr of sample increases, the ions increase in instability so fewer number of whole isomer molecules are detected
***READ FRAGMENTATION PEAKS + M/Z FROM CHEM NOTES 2***
***READ FRAGMENTATION PEAKS + M/Z FROM CHEM NOTES 2***