Electron Transport Chain
1/46
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
47 Terms
At this point, where are we with Metabolism? Where in the mitochondria are we
Glycolysis Done
TCA Done
From TCA: 3 NADH, 1 FADH2, 1 ATP/GTP
PER Acetyl-CoA (each doubles with PER glucose)
Inside the Mitochondrial Matrix
How are electrons transported with carriers and with other things?
With NADH and FADH 2: Donated as an electron pair (2 electrons)
What is the overarching thing happening in the ETC?
Energy transformed from Bond energy into a proton gradient (in the intermembrane space)
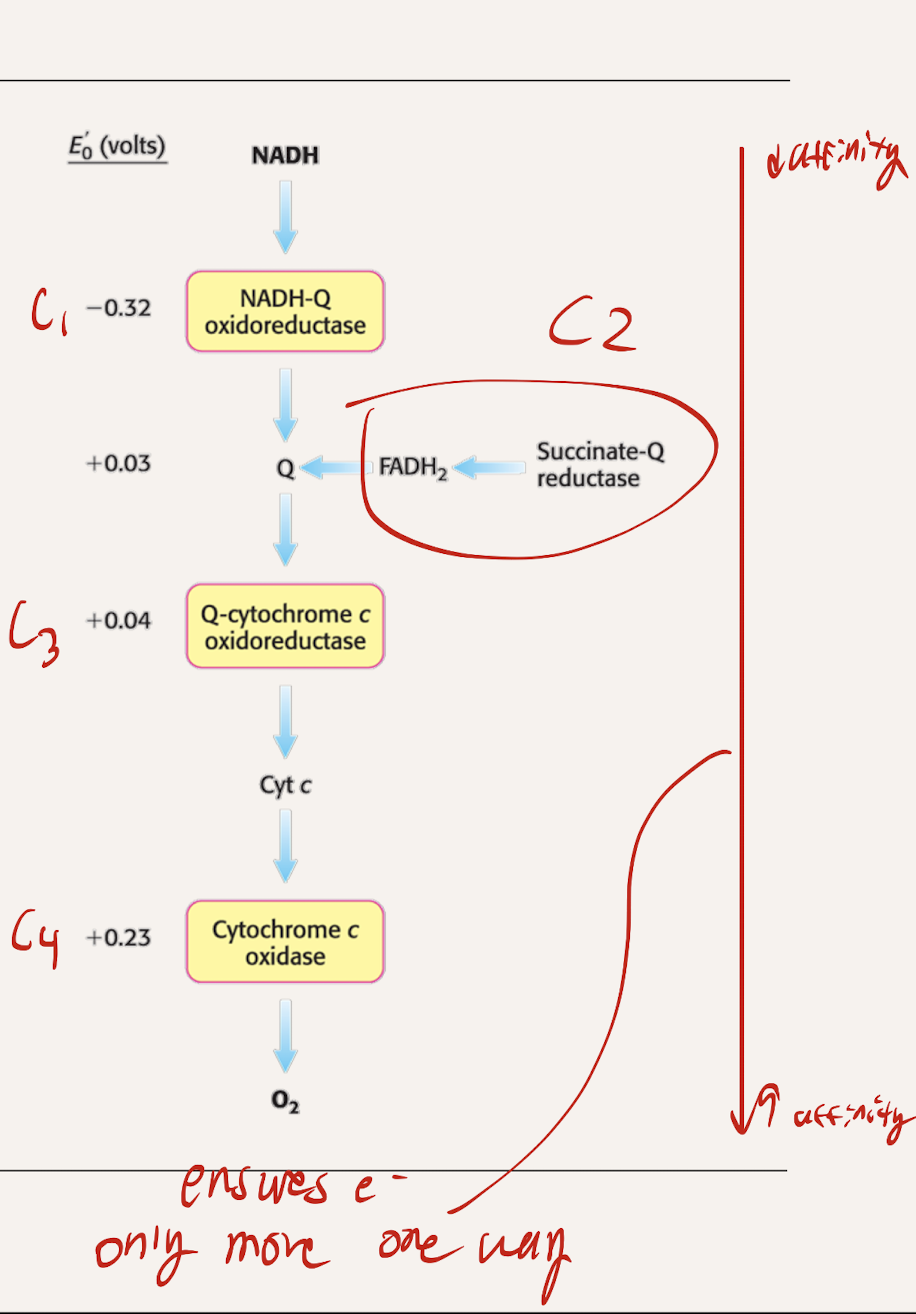
What are the 4 Protein complexes? Which ones are intermembrane proteins, which ones are embedded within the intermembrane
C1 → NADH-Q oxidoreductase
C2 → Succinate-Q reductase
SAME as Succinate Dehydrogenase from TCA
C3 → Q-cytochrome C oxidoreductase
C4 → Cytochrome C oxidase
Intermembrane: C1, C3, C4
embedded within membrane: C2
What is the path that NADH takes through the complexes. FADH2?
NADH: C1 → CoQ → C3 → C4 → O2
FADH2: C2 →CoQ → C3 → C4 → O2
Why do electrons flow in the direction of Compelx 1 to complex 4?
Complex 4 → highest reduction potential (most positive)
Complex 1 → Lowest reduction potential (most negative)
Negative RP → low affinity (wants to give up electrons)
Positive RP → high affinity (wants electrons)
Downhill flow from negative to positive releases energy
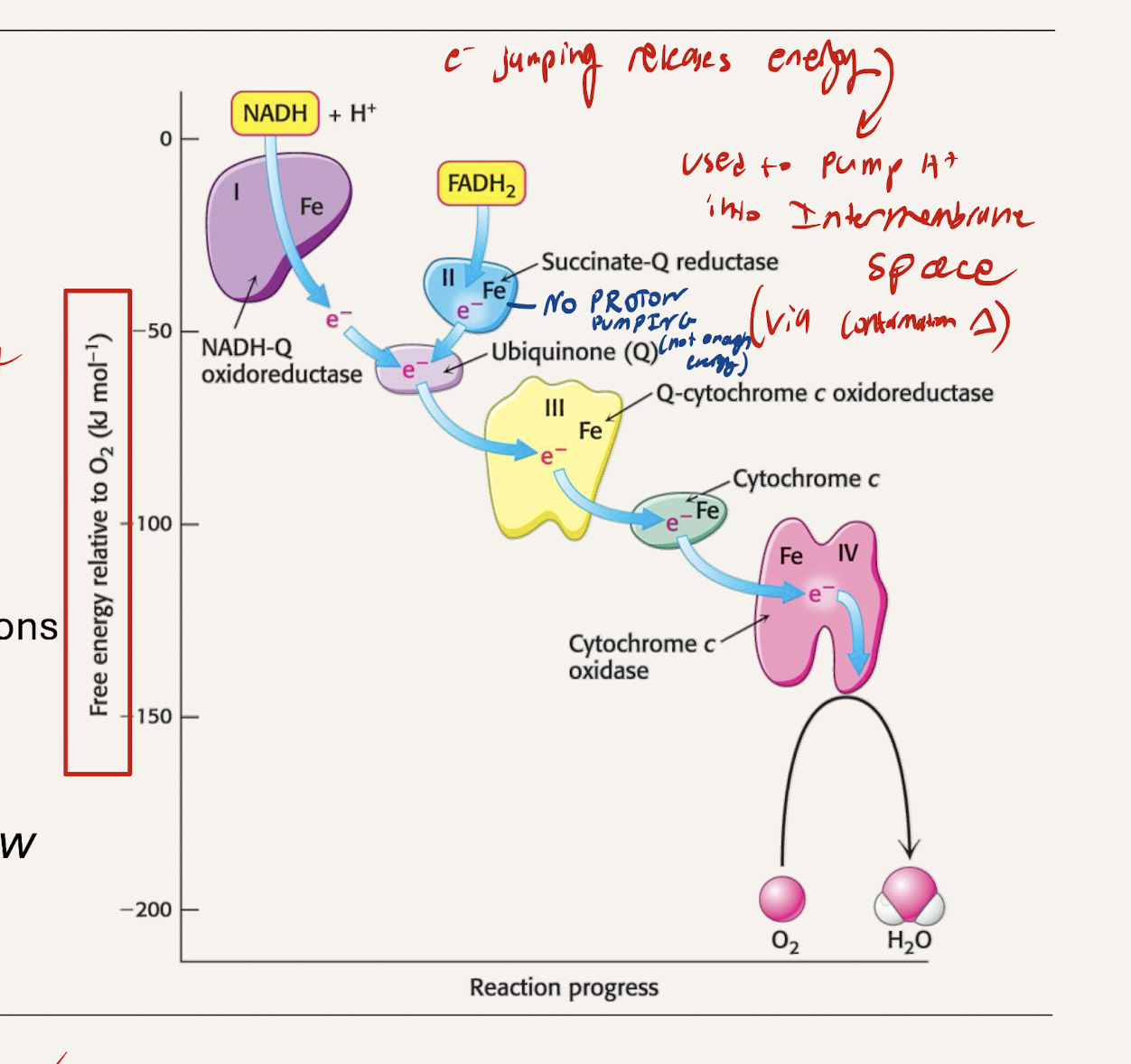
What is teh energy released from electrons jumpoing to lower RP used for?
Provides energy to pump H+ into the intermembrane space (establishing a gradient)
which protein complexes pump H+, which don’t. Why?
C1, C3, C4 all Pump
large enough drop in energy between each to pump out a proton
C2 does not pump
The difference in reduction potential and energy is not great enough to release energy for pumping.
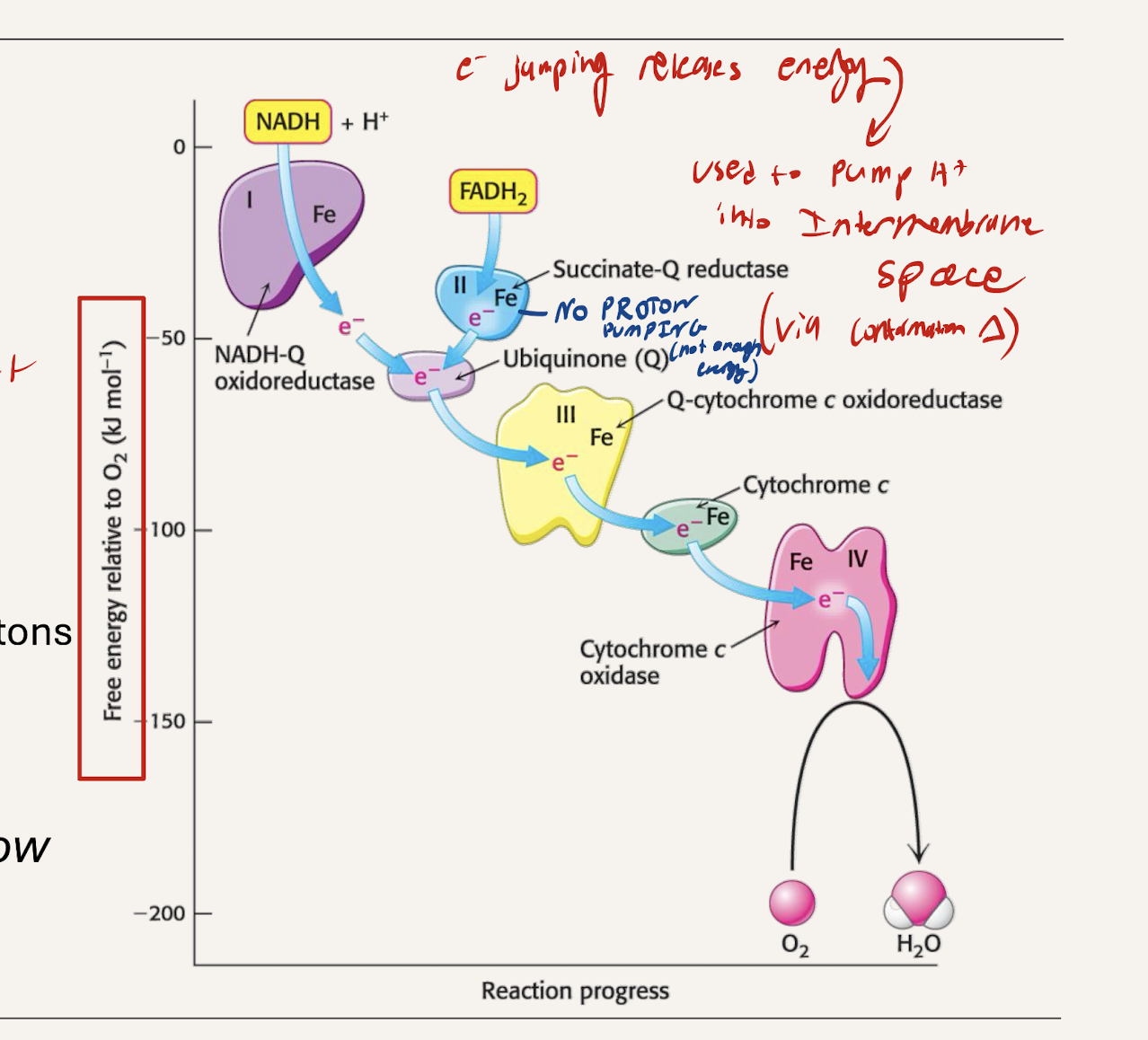
What is teh final desination of the electron? What kind of drop does it have
STEEPEST energy drop (C4 to O2)
Forms H2O (final electron acceptor)
Uses the energy to pump protons
What kind of gradient is the proton motive force?
electrochemical gradient
What does complex 1 contain? Why is each important?
C1 (NADH-Q dehydrogenase) - Where NADH enters
FMN and Itron-sulfur clusters
FMN is the first one to accept electron, then the iron-sulfur clusters
C2 (Succinate-Q oxidoreductase
What does complex 2 contain? Why is each important?
C2 (Succinate-Q oxidoreductase) - Where FADH2 enters
Succinate from the TCA
Succinate dehydrogenase from TCA is attached to C2.
What does complex 3 contain? Why is each important?
Heme groups, Iron-sulfur clusters
Uses Q cycle mechanism (Molecules holding 2 electrons that convert to molecules holding 1 electron)
What does complex 4 contain? Why is each important?
Copper centers and heme a/a3
Final electron acceptor O2
What are the electron carrers used by ETC? Solublity? What complexes do each shuttle electrons between
Coenzyme Q (CoQ)
Fat soluble, moves within the membrane
Shuttles electrons from C2→C3 or C1→C3
Ubiquinone (Q) form or Ubiquinol (QH2) form
Cytochrome C
Water soluble, moves on outer surface of the membrane
Shuttles electrons from C3→C4
Since FADH2 does not pass trhough complex 1 and instead starts at 2, what does that mean comparing with NADH
FADH2 passes through two complexes that pump H+ (C3 and C4)
NADH passes through three complexes pumping H+ (C1, C3, C4)
NADH results in more ATP product b/c it pumps more H+ (since only NADH uses C1 and FADH2 doesn’t)
NADH → 2.5 ATP
FADH2 → 1.5 ATP
Going off last flashcard, how much H+ does each complex pump with one electron pair donation form NADH and FADH2
C1 → 4 H+
C2 → 0
C3 → 4 H+
C4 → 2 H+
How many hydrogens per electron pair for NADH and FADH2 paths
NADH: 10 H+ per pair
FADH2: 6 H+ per pair
What is the structure of NADH-Q Dehydrogenase (C1)
L shaped structure
45 subunits (very large)
Transmembrane protein
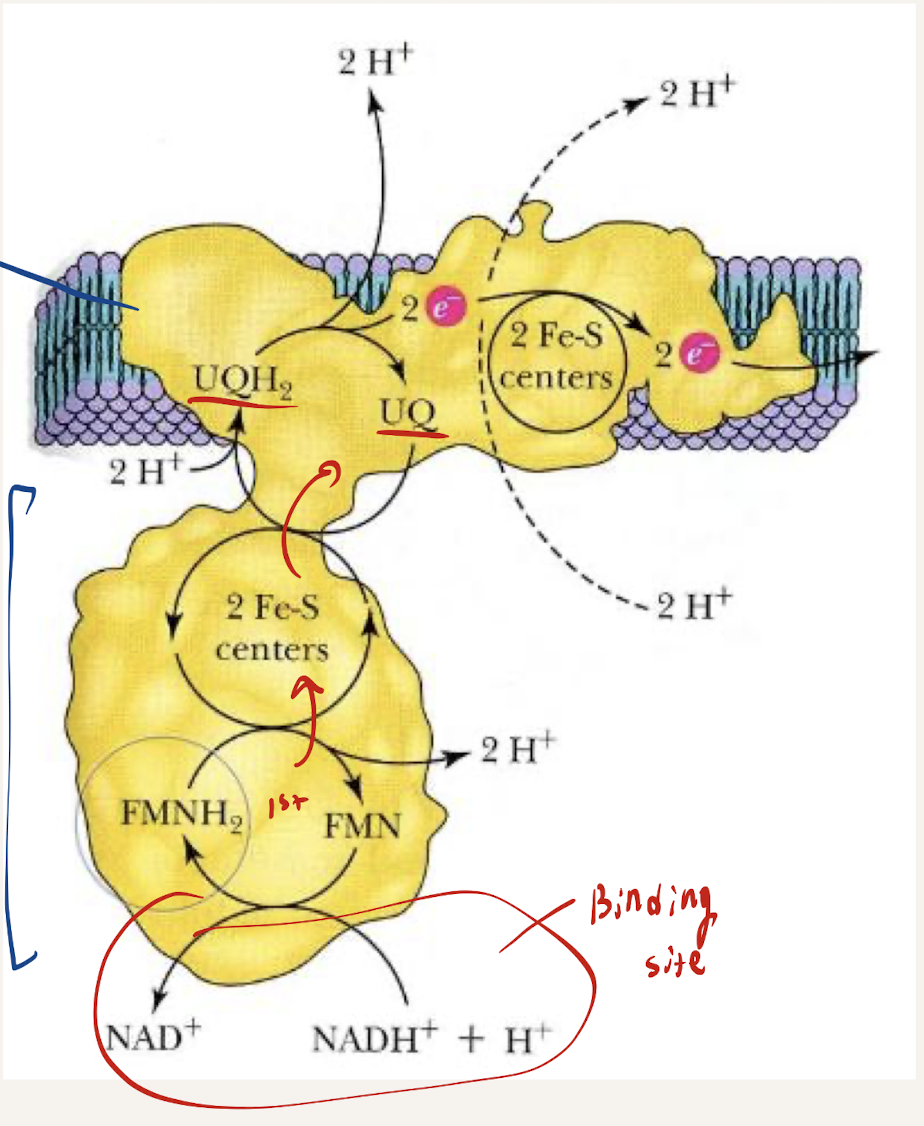
What is the path of electrons within C1 (ELECTRON RELAY). How does electron jumping from each affect the pumping of H+
Electron Relay - NADH donates e- to FMN (Flavin mononucleotide) → FMN donates to Iron-sulfur clusters, Fe-S) → Fe-S donates to CoQ (ubiquinone) → e- flows to the next complex
Jumping bwetwee each causes a ratchet conformation change, releasing a proton from matrix to the IM space
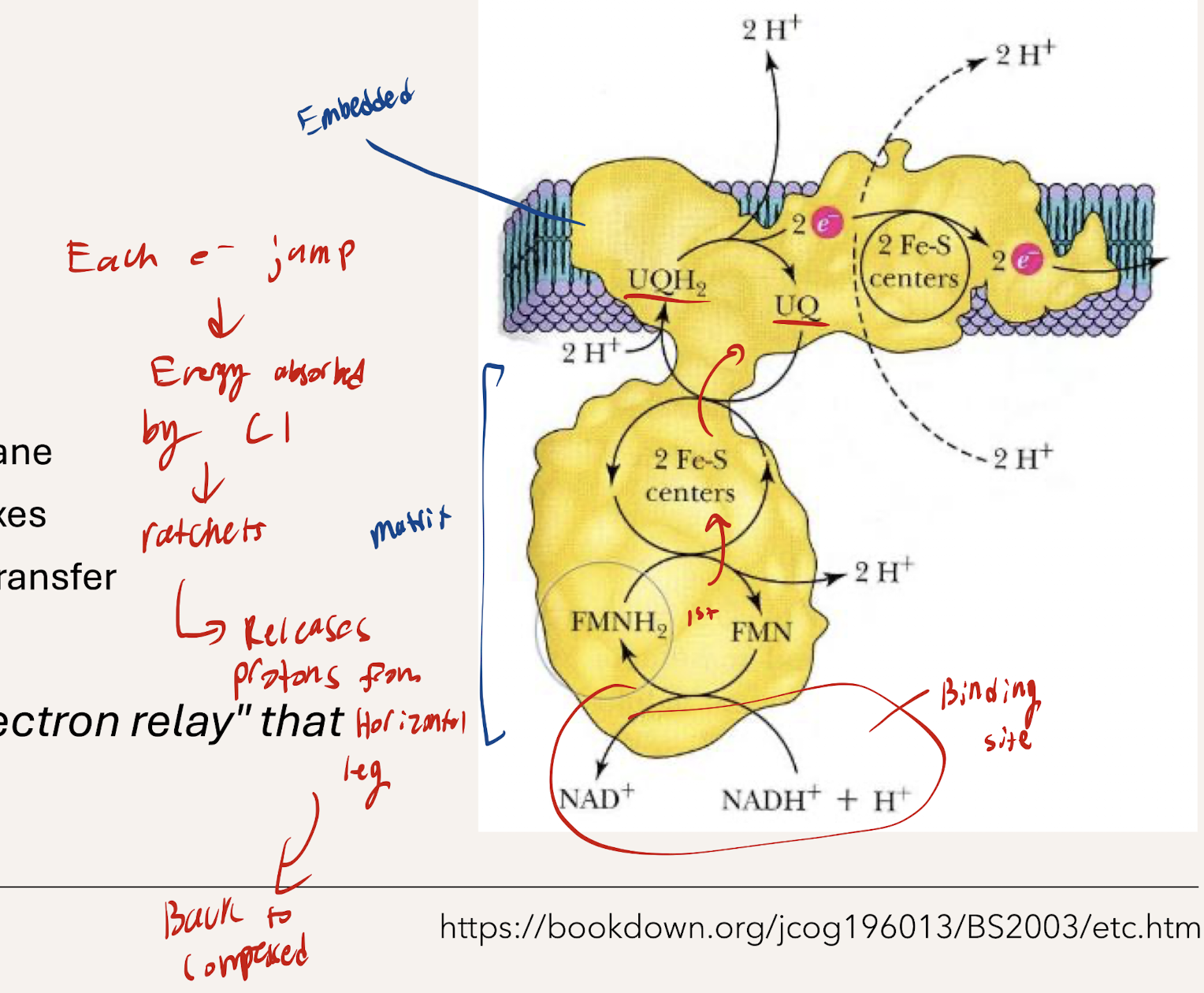
What is the function of Succinate-Q Oxidoreductase/Succinate Dehydrogenase (C2)
DOES NOT pump protons. Within Intermitochondrial membrane
Start point for FADH2
Used for TCA nad ETC
Prevents reactive O3 from forming
What is the path of electrons within C2. What does the enzyme overall show
Succinate → FAD
FADH 2 donates e- to Iron sulfur clusters (3 Fe-S centers) → donate to Heme b → Donate to CoQ (whcih then transports to the next complex)
Evolution integrateed metabolic pathways with TCA and ETC. Same enzyme doing two important functions
What does Complex 3 need to do with the electron carriers? What’s the problem that arises?
Needs to transfer electrons from CoQ (ubiquinol now after having e- donated to it from C1 and C2) to CytC
CoQ carries 2 electrons. CytC only carries 1 electron
Why can’t electrons be simply transferred between CoQ and CytC?
Since CoQ is 2e- and CytC is 1e-, direct transfer is difficult and would waste energy and reduce efficiency.
Fized by the Q cycle
What’s the splitting of electrons called
Bifurcated electron flow
Narrate what happens with Step 1 of the Q cycle
Ubiquinol (QH2) and Ubiquinone (Q) from the Q pool are used. QH2 from C1 or C2 enters. One e- is donated to CytC, reducing it, and then it proceeds to C4. The other e- gets donated to a (Q) in C3 creating a reactive Qi- radical. 2H+ get pumped out
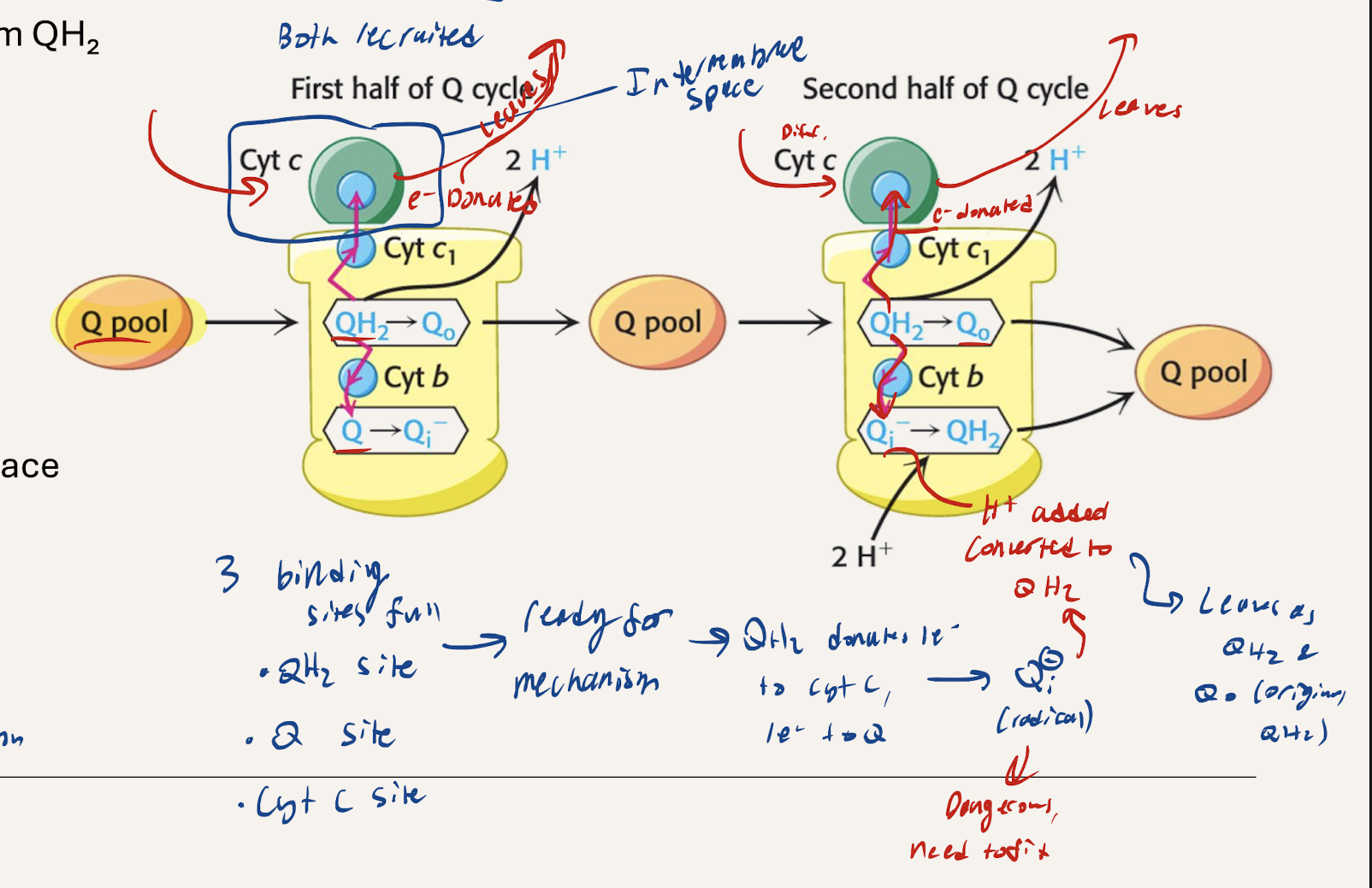
Narrate what happes with Step 2 of the Q cycle
Ubiquinol (QH2) and Ubiquinone (Q) from the Q pool are used. Another QH2 from the Q pool enters, donates 1e- to cytochrome C (reducing it) which then proceeds to C4. The other e- gets donated to the reactive radical Qi- and 2H+ get added as well, converting it to QH2. The QH2 rejoins the Q pool. 2 H+ get pumped out to the intermembrane space
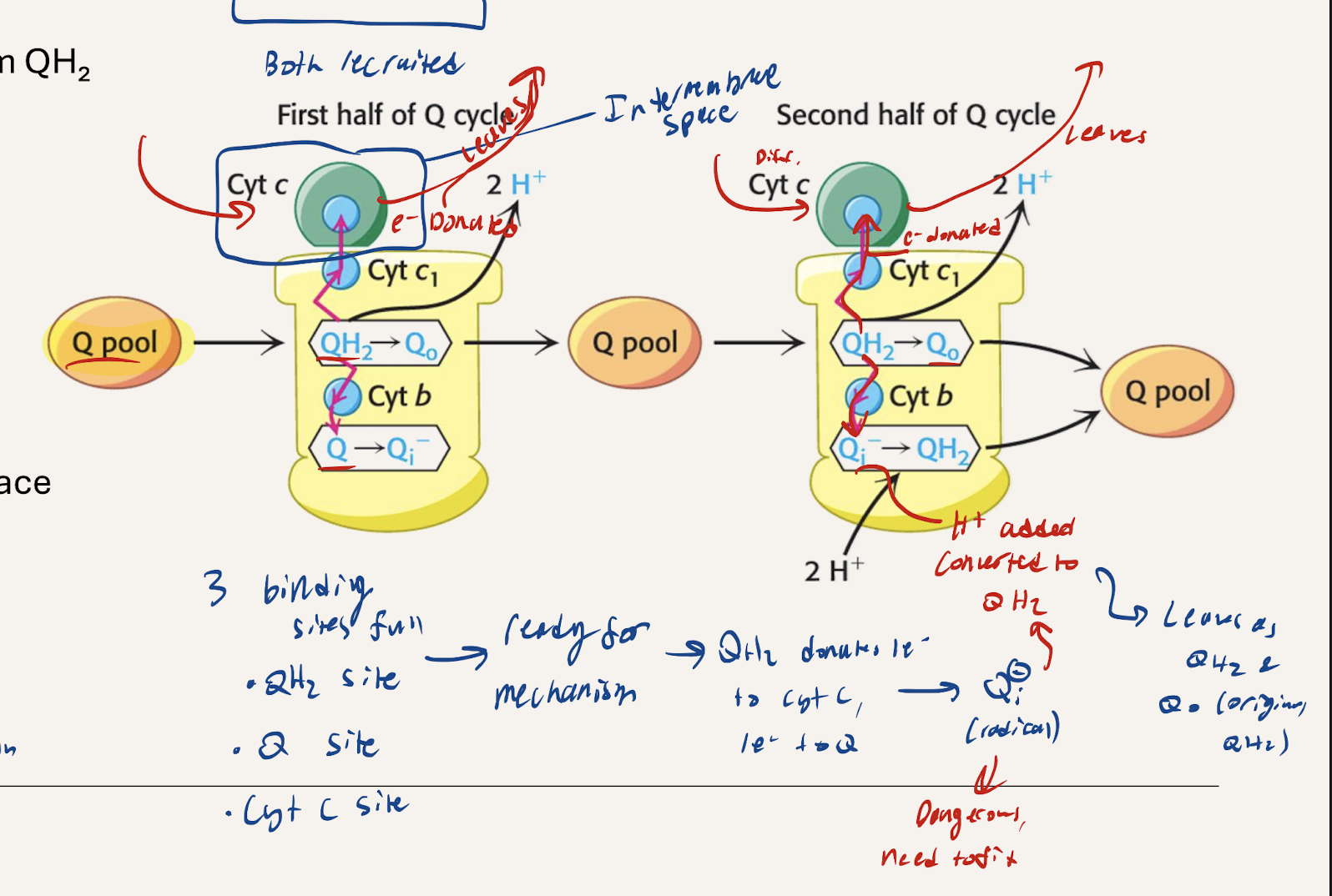
What’s the overall summary of the Q cycle. Net QH2?
2H+ pumped out in each step (4 total)
Electrons transfer from matrix to the intermembrane space
NET 1 QH2 spent (2 QH2 used, 1 made).
4 e- per pair pumped out through C3 → runs to completion once
Why is oxygen the final electron acceptor beyond C4?
Highly electronegative so a strong driving force.
Forms a stable non-toxic product with water (why flourine isn’t used).
Provides renewable final electron acceptor
O2 + 4H+ + 4e- → 2H2O
How do electrons interact with and donate electrons to C4?
1st Cytochrome C binds to complex and donates e- → Iron → Copper.
Distance between iron and copper just enough that O2 can diffuse betwen when electrons are passing anf form peroxide bridge to do the reaction forming water.
2nd Cytochrome C comes in and donates electrons which go up until Iron where is stays because Cu occupied with the 1st electron until peroxide bridge is broken (previous reaction concludes) so the next electron can be used for the reaction.
causes 4H+ to be released with 4 CytC
Represents Ratchet Mechanism
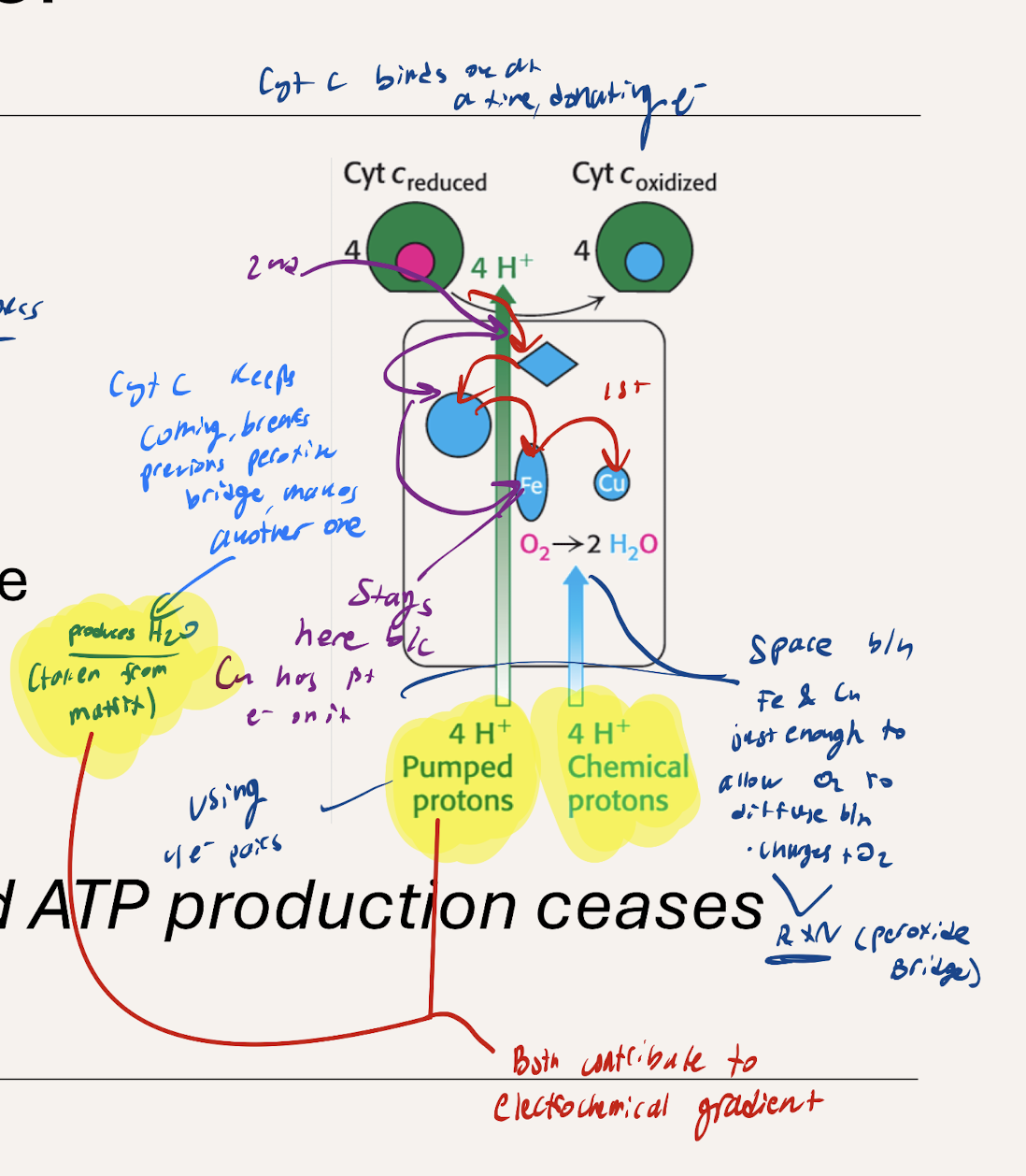
What does CytC with electrons coming over and over again cause?
H2O produced and 4H+ is pumped across into the intermembrane space with 4 CytC, contributing to the electrochemical gradient.
How many Hydrogens pumped out for every electron pair (2e-)
C1 → 4 H+ per 2e
C3 → 4 H+ per 2e
C4 → 2 H+ per 2e
How much H+ for one NADH? How much for FADH2. How much ATP for each?
One ATP needs 4H+
NADH: 10H+
10/4= 2.5 ATP per NADH
FADH2: 6H+
6/4= 1.5 ATP per FADH2
What are possible harmful biproducts of Oxygen metabolism?
Reactive Oxygen species
Superoxide ion (O2-)
two O2- → Hydrogen peroxide (H2O2)
H2O2 + iron presence → Hydroxyl radival (OH)
MOST damaging (b/c very negative can distrupt a lot fo rxns)
How are ROS formed?
Partial reduction of Oxygen dyring electron transfer
1-2 or 0.1-0.5% of oxygen undergoig ETC will become ROS
Why are ROS so dangerous?
highly reactive disrupting Proteins, DNA, lipids
accumulation → cell and tissue death
What enzyme families protect against ROS? Specific enzymes of each?
Antioxidant Enzymes
Superoxide Mutase
Catalase
Glutathione System
Glutathione peroxidase
Glutathione reductase
What does each enzyme do/how does it function
Antioxidant Enzymes
Superoxide Mutase
Converts Superoxide to H2O2
Catalase
Converts H2O2 to H2O + O2
O2- —Superoxide Mutase→ H2O2 —Catalase→ H2O + O2
Glutathione System
Glutathione peroxidase
2 Glutathione (GSH) + H2O2 → H2O + O2 + oxidized Glutathione (GSSG)
Glutathione reductase
GSSG + NADPH → GSH
2 GSH + H2O2 —Glutahione peroxidase→ H2O + O2 + GSSG
GSSG + NADPH —Glutathione Reductase→ GSH
What is an inhibitor of each of the ETC Comlexes (1, 3, 4)
NADH-Q dehydrogenase (1)
Rotenone (incesticide)
Q-Cytochrome C oxidoredctase (3)
Antimycin A
Cytochrome C oxidase (4)
Cyanide
All inhibitors stop ATP production by disrupting electron flow
What does Rotenone inhibit for NADH-Q dehydrogenase (1)
Binds to Ubiquinone (Q) binding site blocking NADH oxidation
What does Amitmycin A inhibit for Q-Cytochrome C oxidoreductase (3)
Binds to Qi site of Q cycle
Blcoks electron transfer to cytochrome C
What does Cyanide inhibit for Cythchrome C oxidase
Binds in place of oxygen blocking oxygen reudction
How do mitochondrial diseases affect the body? How do they arise”
Affect tissues that need a lot of energy (muscle, brain, heart).
Muscle weakness, exercice intolerance, neurological problems
Result from mutations in electron transport proteins or mitochondrial DNA
Why are mitochondrial diseases so dangerous?
Energy crisis (cells can’t reach ATP demands)
Progressive Damage - Lactic Acid Builds up; energy-starved tissues deteriorate over time
No effective treatments
What is MELAS sydnrome? What does it stand for
Mitochondrial Encephalomyopathy Lactic Acidosis Stroke-like episodes
What is MELAS caused by?
Mutations in mitochondrial tRNA genes → innefective protein synthesis in mitochondria → ETC doesn’t work
mainly affects the brain and muscle b/c of high ATP needs
Symptoms, treatment, and prognosis of MELAS?
Symptoms Seizures, muscle weakness, visoin loss, stroke-like episode
Treatment: Supportive care (no cure)
CoQ10, vitamins, no metabolic stress
Prognosis: Progressive decline. Fatal for youngins