Heat Capacity
1/29
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
30 Terms
Molar Heat Capacity at Constant Volume
The amount of heat required to raise the temperature of 1 mole of a substance by 1 K at constant volume.
C_V = (1/n)(Q/ΔT)
Mathematical representation of Molar Heat Capacity at Constant Volume
Q = n(C_V)ΔT
Q = ?
Constant-Volume Heating (Ideal Gas)
At constant volume, the gas does no work (W = 0), so any heat added changes only the internal energy
ΔEint = Q (at constant V)
Mathematical representation of the Constant-Volume Heating (Ideal Gas)
Eint = (3/2)(nRT)
Internal Energy of a Monoatomic Ideal Gas
ΔEint = (3/2)(nRΔT)
Change in the Internal Energy (Monoatomic Ideal Gas, Constant C_V)
From ΔEint = Q = n(C_V)ΔT and ΔEint = (3/2)(nRT): C_V = (3/2)R
Molar Heat Capacity of a Monoatomic Ideal Gas at a Constant Volume
(3/2)R
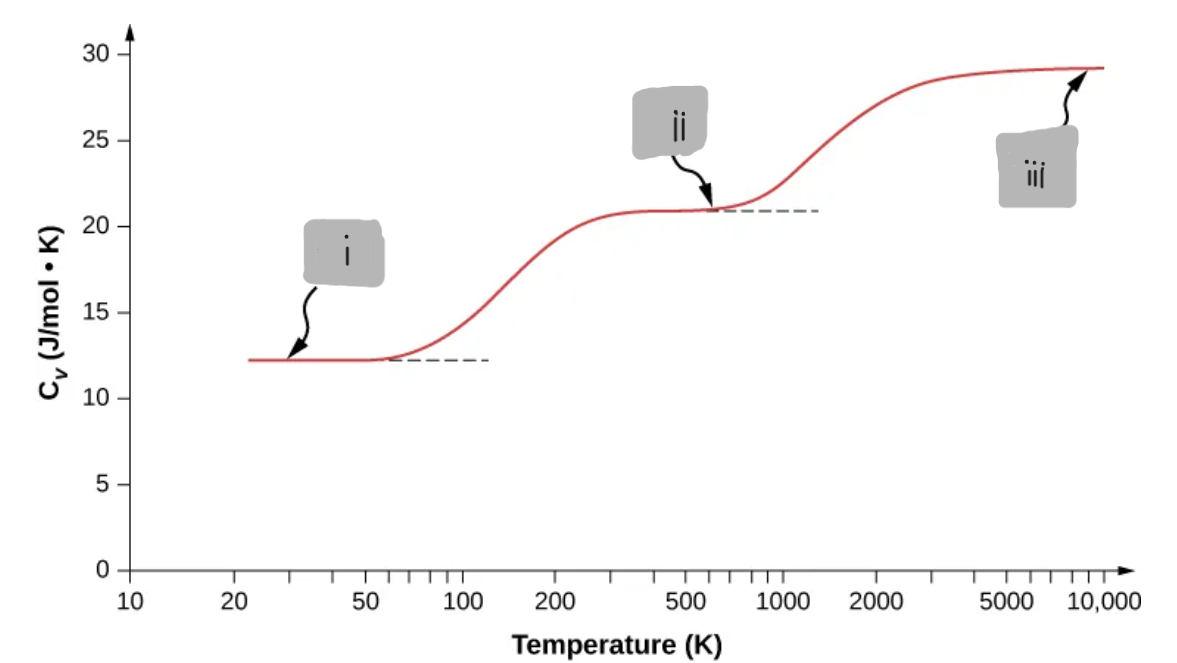
(i)
(5/2)R
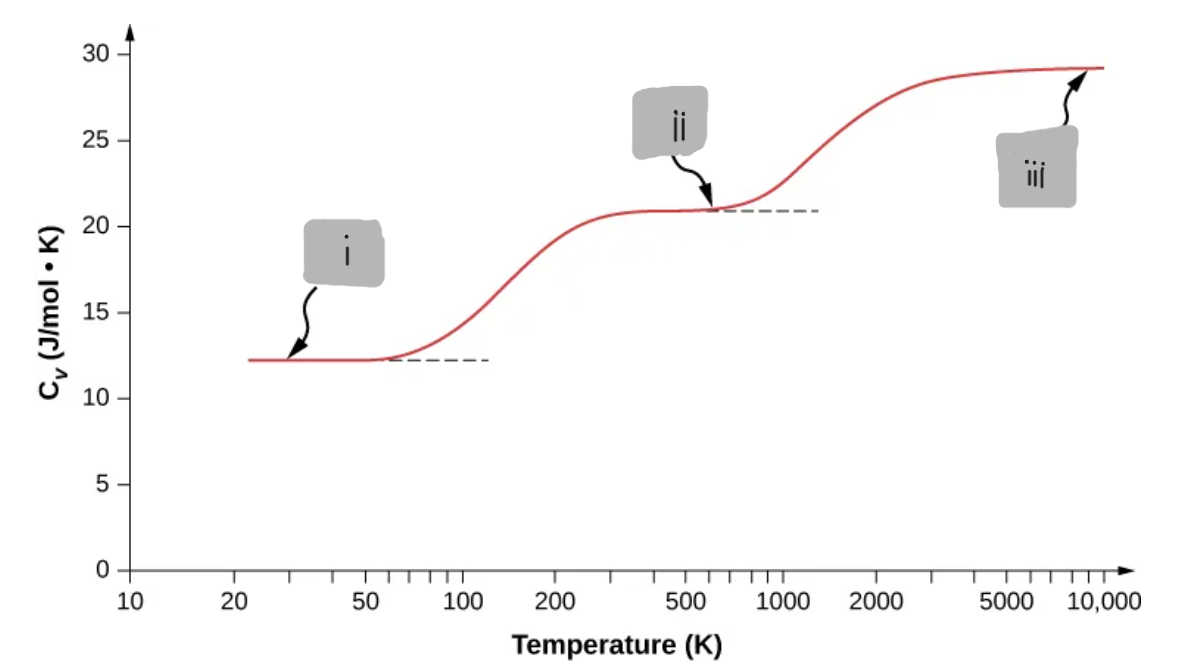
(ii)
(7/2)R
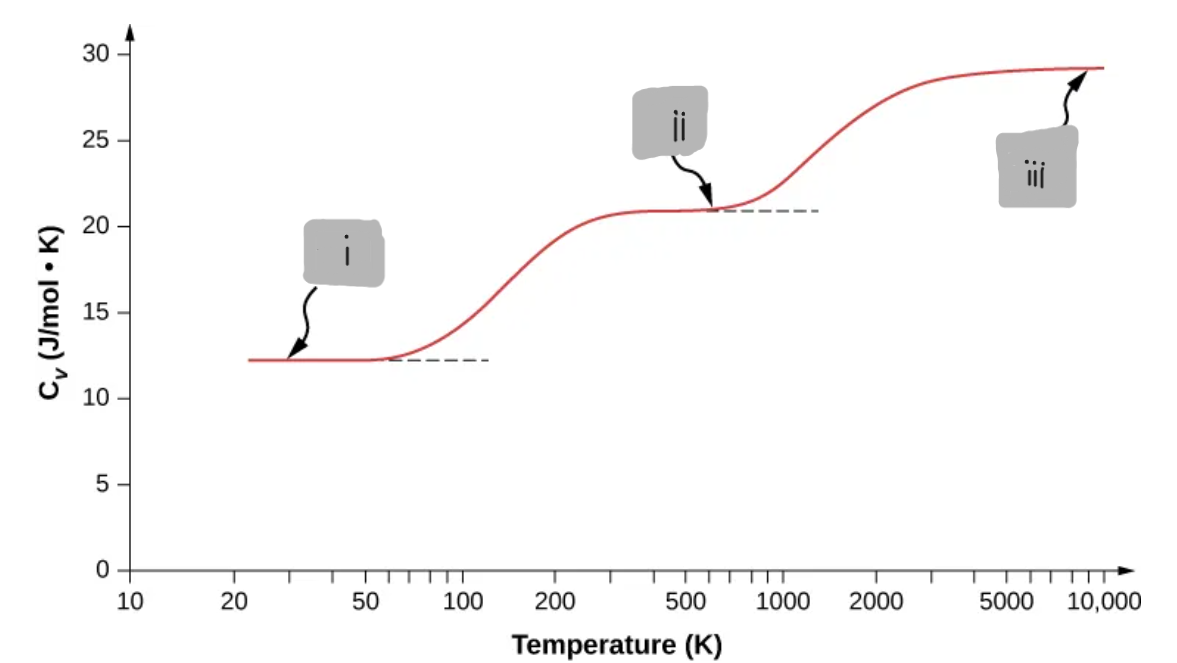
(iii)
Degree of Freedom (d)
An independent possible motion of a molecule that store energy
e.g., translation and in x, y, z; rotation; vibration
3
A monoatomic gas has _ translational degrees of freedom (motion along x, y, z).
K̅ = (1/2)mv̅^2 = (3/2)(k_B)T
Average Translational Kinetic Energy (per molecule)
As (1/2)(k_B)T contributed by each of the 3 translational degrees of freedom
Average Translational Kinetic Energy (per molecule) can also be seen as what?
C_V = (d/2)R
Molar Heat Capacity in Terms of Degrees of Freedom for an ideal gas with d active degrees of freedom.
Equipartition Theorem
In classical thermodynamics, at thermal equilibrium,
energy is shared equally among all active degrees of freedom
Each degree contributes (1/2)(k_B)T per molecule to the internal energy
C_V = (d/2)R
for an ideal gas
E_int = (d/2)nRT
Internal energy of an Ideal gas with d degrees of Freedom
Degrees of Freedom in a Diatomic Molecule (Classical View)
3 translational (x, y, z)
2 rotational (around axes perpendicular to bond axis)
2 vibrational (1 kinetic + 1 potential along the bond)
Total classical: d = 7
Quantum Activation of Degrees of Freedom (Diatomic Gas)
Due to quantum mechanics, we know that not all degree of freedom are active at a given temperature. They “turn on” in steps as temperature increases.
Diatomic Gas at Very Low Temperatures (<~60K)
Only translational motion is active
d = 3
C_V = (3/2)R
Behaves like a monoatomic gas
Diatomic Gas at Room Temperature (≈ 300 K to ≈ 600 K)
Translations + rotations active
Vibrations are mostly frozen out
d = 5
C_V ≈ (5/2)R
Diatomic Gas at Very High Temperatures (≈ 3000 K and above)
Translations + rotations + vibrations
All are active
d = 7
C_V ≈ (7/2)R
Polyatomic (Nonlinear) Gas at a Typical Room Temperature d
Usually has 3 translational motion + 3 rotational degrees of freedom active
d ≈ 6
C_V ≈ 3R
Additional Vibrational modes become active only at higher temperatures
diatomic, polyatomic
For _________ and __________ gases, C_V depends on temperature, because different degrees of freedom become active at different temperatures (step-like behavior)
Spring Model of a Solid
Atoms in a solid can be modeled as masses connected by springs to neighboring atoms, allowing vibrations in x, y, and z.
Degree of Freedom for an Atom in a Solid
Each atom has:
3 kinetic (vibrations along x, y, z)
3 potential (spring potential in x, y, z)
Total: d = 6
C_V = (d/2)R = (6/2)R = 3R
Predicted Molar Heat Capacity of a Solid (Equipartition) using d = 6
Law of Dulong and Petit
Empirical rule stating that many solid elements have
Molar Heat capacities close to C_V ≈ 3R
at ordinary temperatures,
Consistent with the 6 degrees of freedom per atom in the spring model
Limitations of Dulong-Petit Law
It fails at low temperatures and for some light elements (like Be and C) also it fails for some heavier metals, because quantum effects reduce the number of active degrees of freedom.