OCR A 2.1.2 & 2.1.3 Amount of Substance, Compounds, Formulae and Equations 1"
1/18
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
19 Terms
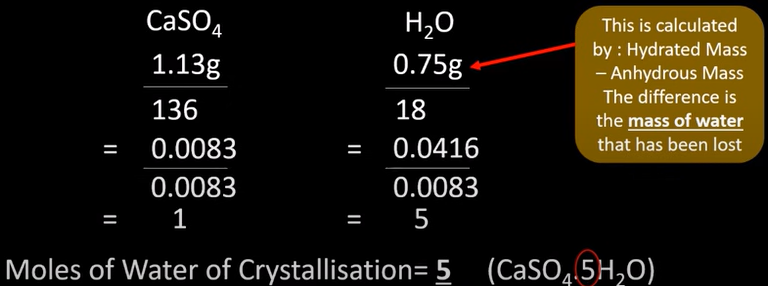
how to calc water of crystallisation (experiment)
-measure mass of hydrated salt
-heat until no more water left
-anhydrous salt left
-measure mass of this
-the find mass of water and divide all known masses by the mr to find moles
-find molar ratio

ionic equation steps
-split aqueous substances
-cross out ions on both sides
mole
-way of measuring the amount of a substance
-6.02 ×10²³
-1 mole of Fe contains 6.02 ×10²³ atoms of Fe
how to find number particles from moles

mole, mass, mr equation

moles in sol equation

cm to dm
divide by 1000
ideal gas equation
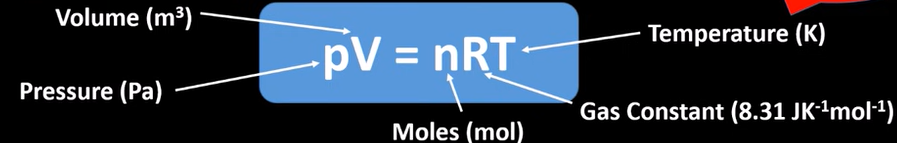
standard conditions
-298K
100kPa
aqueous meaning
dissolved in water
empirical formula
simplest whole number ratio of elements in a compound
percentage yield

atom economy
-how efficient a reaction is
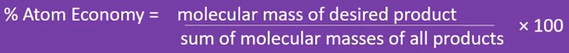
importance of atom economy
high atom economy:
-produce less waste
-less by-products; less time & money spent separating these from desirable product
-raw materials used more efficiently; more sustainable
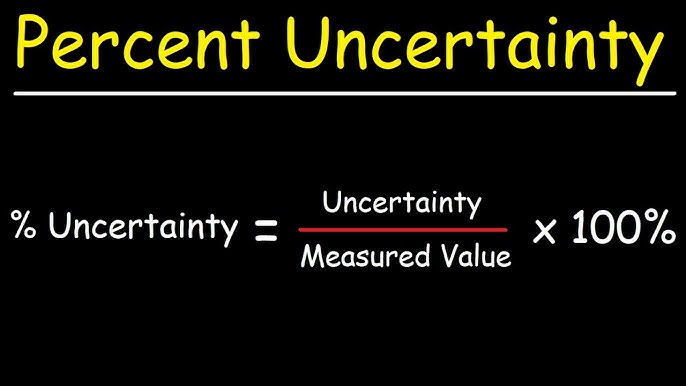
percentage uncertainty formula
how to reduce percentage uncertainty
-more sensitive weighing scale (more dp)
-larger mass
how to find volume of gas from moles
-moles x 24(dm)/24000(cm)
molecular formula
actual number of atoms of each element present in a molecule