BIOG 1440 Prelim 1 -- Cornell Fall 2025
1/34
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
35 Terms
Explain the role of water as the solvent of life, and how the hydrophobic effect leads to self-assembly of biological membranes.
"Water---due to its hydrogen bonds and partial charges---is able to covalently attract lone pair electrons. This allows water to dissolve many solutes, including ionic molecules and polar large macromolecules, by separating the individual charged or polar components of the other molecule. In terms of the phospholipid bilayer---composed of hydrophilic glycerol heads and hydrophobic, nonpolar fatty acid chains---individual phospholipids orient themselves into a phospholipid bilayer to exclude water from their internal regions and to form the most energetically efficient structure."
Describe membrane bilayer organization within the context of the fluid mosaic model.
"The membrane bilayer is made up of essentially equal parts phospholipids and carbohydrates/proteins. The molecular components of the membrane---including both intramembrane and on the exterior of the membrane---are fluid, allowing components to move laterally. While some proteins may be anchored in place, the hydrogen bonds that compose a majority of the membrane allow for this level of fluidity."
Describe how variation in membrane lipid composition affects the fluidity and integrity of membranes.
"Lipid composition is the primary determinant of membrane fluidity. However, it is also important to note that for eukaryotes, cholesterol also regulates fluidity. Based on external temperature, the cholesterol changes how packed together the fatty acid groups are, changing how easily other membrane components can move laterally within the complex. The saturation of the phospholipids also dictates membrane fluidity. Unsaturated fatty acid groups mean that they have a double bond and less than the regular amount of carbon chains (saturated is vice versa). This causes saturated fatty acid groups to appear straight, and unsaturated to appear bent. When saturated, fatty acid groups are easily able to pack together tightly, thereby decreasing membrane fluidity. Conversely, when unsaturated, fatty acids cannot pack tightly and thereby allow for large amounts of lateral movement."
Explain how membrane proteins interact with the membrane and describe their many functions.
Membrane proteins have 6 distinct functions: transport of water/nutrients, cell to cell adhesion, cell to cell recognition, attachment to cytoskeleton, enzymatic activity, and signal transduction. Cells use active or passive transport, via transmembrane proteins, to transport water and other molecules across the membrane. Membrane proteins can act as complementary proteins, allowing them to bind with carbon chains in another membrane and undergo cellular recognition. Proteins can also engage in intracellular adhesion (gap junctions in eukaryotes and plasmodesmata in prokaryotes). Proteins also can bind to the extracellular matrix via integrins (a type of intracellular protein). Finally, proteins can serve as enzymes which are activated by extracellular ligands, thereby inducing a signal cascade and inducing signal transduction.
Define osmosis and its relationship to hypertonic and hypotonic environments.
Osmosis is the movement of water across a semipermeable membrane in the direction of high water concentration to low water concentration (low solute concentration to high solute concentration). Water can diffuse via aquaporins or simple diffusion, and its direction depends on the relative concentrations inside and outside of the cell membrane. In hypertonic environments, defined by a higher solute concentration outside the cell than inside, the water will rush out in order to maintain equal concentrations, causing the cell to shrivel. In hypotonic environments, defined by a lower solute concentration outside the cell than inside, the water will rush into the cell, causing it to become lysed. In isotonic environments, the net movement of water is 0; however, water is still leaving and entering the cell. These 3 states are essential to turgid pressure, which helps plant and bacterial cells maintain their cellular structure (turgid, flaccid, plasmolyzed).
Describe the difference between facilitated diffusion and active transport.
Facilitated diffusion does not require energy, whereas active transport uses ATP or a chemical gradient. Facilitated diffusion moves molecules in the direction of their concentration gradient, and relies on conformational proteins that allow specific molecules through. Active transport also utilizes conformational transmembrane proteins, but sends molecules against their concentration gradient, usually to maintain an electrochemical gradient. Examples of facilitated diffusion include aquaporins, semiSWEET proteins (glucose), and Glut-5 (fructose). Prime example of active transport are cotransport and the Na+/K+ pump.
Summarize how energy is used to drive active transport via the alternating access model and can lead to the formation of membrane potential.
Active transport requires energy, which comes in two primary ways: ATP and cotransport via the electrochemical gradient. P-type ATPases use ATP hydrolysis to transport 3 Na+ ions out of the cell and 2 K+ ions back into the cell, against their respective concentration gradients. P-type ATPases use the alternate access model as they change between two states: Na+ external state and K+ internal state. By moving ions inside and outside the cell, the sodium potassium pump works to conserve its membrane potential, which is an electrochemical gradient with the intracellular space being negatively charged (less K+) and the extracellular space being positively charged (more Na+). In plants and microbes, this electrochemical gradient is called the Proton Motive Force (PMF) and can be used to do work around the cell.
Describe bulk transport
Bulk transport refers to the processes of endocytosis and exocytosis, which moves molecules inside and outside of the cell membrane. Exocytosis uses vesicles to transport waste/neurotransmitters/insulin out of the cell. Endocytosis refers to 3 functions: phagocytosis (food being taken up via vacuoles), pinocytosis (cellular drinking using vesicles), and receptor-mediated endocytosis (a signal command telling a vesicle to capture nearby molecules).
Describe features of a biochemical reaction, including substrates, products, what defines a
spontaneous reaction according to the G, and what defines equilibrium.
In a biochemical reaction, reactants combine to form products. Each reaction has an equilibrium constant (KEQ=[AB][A][B]) which is dependent on the comparative concentrations of the reactants and products as well as the change in gibbs free energy (G=H-TS). Gibbs free energy is equal to enthalpy - entropy. Reactions that are spontaneous require no energy and have a -G, whereas reactions that require an input of energy have a positive G. If G0, then KEQ1, and the products are favored over the reactants at equilibrium. If G0, then KEQ1, and the reactants are favored over the products at equilibrium. Reactions that have a -G are exergonic (energy releasing), while those that are G+ are endergonic. Spontaneous, exergonic reactions happen spontaneously (without energy input), however, they can happen at very different rates. The kinetics of a reaction are determined by Kf and Kr. All reactions proceed until they reach equilibrium. At equilibrium, Kf = Kr. KEQ=KfKr, meaning that it is the ratio of forward and reverse reaction rates.
Explain how enzymes act as catalysts to increase the rate of reactions by reducing the
activation energy.
Enzymes catalyze reactions by reducing the amount of activation energy needed for a reaction to Occur and by increasing the stability of the transition state. Enzymes are not part of the reactants Or products of a reaction, but instead help to speed up the rate of reaction. Although they affect Activation energy, they do not affect the total change in G of a reaction.
Describe structural features of enzymes, including active and allosteric sites.
Enzymes work via induced fit, meaning that the binding of the substrate changes the shape Of the enzyme. All enzymes have an active site, and many can have an allosteric site. Enzymes Use a chronological process to function: creating a high local concentration of substrates; Orienting the substrates correctly; changing shape; using acids, bases, or other cofactors To speed up a reaction; and using binding energy to stabilize the transition state. Throughout Their function, they transition from enzyme, to the enzyme substrate complex, to the enzyme product Complex, back to a regular enzyme.
Distinguish competitive and non-competitive enzyme inhibitors.
Competitive enzyme inhibitors are molecules with similar structure to substrates. They bind to The active site of an enzyme, competitively inhibiting the enzyme by no longer allowing the regular Substrate to bind. Non-competitive inhibitors bind to the allosteric site, which is in a different
Location than the active site. Non-competitive inhibition works by changing the structure of The enzyme when the inhibitor binds, thereby no longer allowing regular function of the active site.
Describe mechanisms of feedback regulation.
There are two types of feedback regulation, positive and negative. Negative feedback utilizes the allosteric site of an enzyme. When too much of a product is being formed, an inhibitor binds the enzyme
And prevents further product formation. Conversely, when too little product is being formed, the Inhibitor deattaches from the enzyme, thus allowing for increased production. This allows for The maintenance of homeostasis. Positive feedback is where the production of a product Stimulates increased production — thus moving production in one level or another and against Homeostasis.
Explain how coupling ATP hydrolysis to an otherwise non-spontaneous reaction enables a
reaction to proceed.
ATP hydrolysis converts ATP to ADP and a free phosphate group. The reaction is highly exergonic, releasing energy that can be used to compensate for an endergonic reaction of equal or less energy demand. Since endergonic reactions are energetically unfavorable and require large energy input,
coupling the two reactions provides the activation energy needed for the reaction to occur.
Identify physical variables that influence the rates of enzyme reactions
All enzymes have an optimal temperature and pH, and there is a bell curve for functionality. Other factors that influence rates include substrate concentrations, enzyme concentrations, and the presence of inhibitors.
Describe how the catabolic reactions of metabolism oxidize organic fuels (e.g glucose) to yield energy (chemical energy in ATP and energy stored in the electrochemical gradient of the PMF).
Cellular respiration involves 4 major processes: glycolysis, pyruvate oxidate, TCA cycle, and Oxidative phosphorylation. This 4 step sequence transforms glucose into 30-38 ATP.
Describe the four major pathways (glycolysis, pyruvate oxidation, tricarboxylic acid (TCA) cycle, oxidative phosphorylation) involved in glucose catabolism. Include in your description the order of the pathways, the inputs and outputs of each pathway, where the pathways occur in cells, and which pathways are the major sources of NADH and ATP. (You don't need to know each intermediate in the pathways, or the enzymes that control them).
Glycolysis is the initial step in cellular respiration, taking place in the cytoplasm. It has two phases, the energy investment phase and the energy payoff phase. Glucose is the main input, while 2 pyruvates, 2ATP, and 2 NADH are the main outputs. Pyruvate oxidation is the second reaction, taking place in the mitochondria. Its main inputs are 2 pyruvates, 2 Coenzyme As, and it produces 2 Acetyl-CoAs, 1 CO2, and 2NADH. The TCA cycle is next, located in the mitochondria. 1 glucose molecule runs the TCA cycle twice. Its inputs are 2 Acetyl-CoAs, 4H20; while its outputs are 4CO2, 6NADH, 2FADH2, 2ATP, and 2CoA. Finally, oxidative phosphorylation has 2 phases and occurs in the inner membrane matrix. The first phase is the ETC, which transports 10NADH throughout the ETC to bind to O2, thus creating H2O. It also pumps 10 H+ ions out of the inner membrane. The second phase of the reaction is chemiosmosis, where the H+ ions diffuse back into the inner membrane through ATPase, thus generating ATP. In all, 10NADH and 25ADP are used to create 25ATP and H2O. This is the main source of ATP in cellular respiration.
Describe the role of molecules that can be reversibly reduced and oxidized as carriers of electrons removed during the catabolism of glucose. Redox cofactors include cofactors such as NAD/NADH and FAD/FADH2, protein-associated metals (e.g. FeS clusters), and membrane-soluble carriers (quinones).
NADH and FADH2 are oxidized throughout the first 3 steps, but are mainly used in oxidative phosphorylation. They each enter the ETC (NADH at complex 1 and FADH2 at complex 2) and pump H+ ions out of the inner membrane before eventually reducing o2 into H20. They are important in establishing the PMF that is used to pump protons back into the membrane through ATPase, thereby creating ATP.
Quinones are another type of electron carrier that play a role in the ETC, shuttling electrons throughout.
Describe how the respiratory chain couples the energetically favorable transfer of electrons from donor to acceptors to the energetically unfavorable pumping of protons against an existing proton gradient (to generate PMF)
NADH and FADH2 transfer electrons to O2 — an energetically favorable reaction. The movement of these electrons is exergonic. Simultaneously, they pump H+ ions out of 4 protein complexes, against their concentration gradients (endergonic). The energy offsets, allowing for this energetically unfavorable movement to occur.
Describe the role of O2 molecules as the terminal electron acceptor (in mitochondria and during aerobic respiration).
The O2 molecules accept electrons from NADH and FADH2, transforming into H20—which is a byproduct of cellular respiration. O2 binds with H+ ions within the mitochondiral membrane.
Define the function of the F1FO-type ATPase and how it is used to displace the ADP + Pi ATP from equilibrium (chemiosmosis)
ATPase is the transmembrane protein that typically pumps Na+ and K+ against their concentration gradients using ATP. In the ETC, ATPase runs in reverse, allowing H+ ions to diffuse back into the inner membrane with their concentration gradient. This energetically favorable movement, spurred by the PMF, allows for the energetically unfavorable transfer for ADP into ATP.
Describe how anaerobic respiration (use of alternative electron acceptors) allows diverse modes of respiration even in anaerobic habitats, and how the diversity of microbial metabolism can contribute to elemental cycling in ecosystems.
Anaerobic respiration uses alternative electron acceptors to oxygen, including Fe(III), Mn(IV), NO3, S, and Fumarate. The molecules are used as a replacement for O2 in the ETC, allowing cells to continue to harness the ATP from that process. All these alternatives are eventually reduced as waste, allowing for elemental cycling.
Describe the steps of fermentation , including how fermentation recycles NAD+, allowing continued glycolysis and ATP formation.
Fermentation is not a form of aerobic respiration, as only glycolysis is performed. Fermentation recycles NAD+ from NADH by forming ethanol or lactate. Ethanol is synthesized by oxidizing NADH and reducing 2 acetylaldehyde, while lactic acid is synthesized by reducing 2 pyruvates. This allows for the cycle to continue, and for the continuous glycotic production of ATP.
Describe how bacteriorhodopsin (in some Archaea) can function to convert light energy directly into PMF to allow ATP synthesis.
Bacteriorhodopsin, a type of photoheterotroph, uses a specific type of function to synthesize ATP. They convert the light energy they receive into facilitated diffusion of H+ ions against their concentration, thereby establishing a PMF. This PMF is then harnessed to produce ATP via chemiosmosis and P-ATPase.
Distinguish between oxygenic photosynthesis and cyclic photophosphorylation as it occurs in bacteria such as Rhodobacter sphaeroides, a purple bacterium.
Cyclic photophosphorylation is a process that involves only one photosystem (not the Z structure). It is different from bacteriorhodopsin because no PMG is created. Instead, PSI is struck by energy, Which excites chlorophyll, donating an electron to the primary acceptor complex. This complex then Creates ferreoxidin, which binds to the cytochrome complex and produces ATP and plastocyanin. Plastocyanin can then reexcite PSI and the entire cyclical process. Cyclical photosynthesis does not Rely on NADPH and can occur with or without oxygen.
Describe the chemical steps that allow light energy to be harvested and used to make ATP and NADPH during the light reactions of oxygenic photosynthesis.
1. P680 of PSII is excited
2. P680* donates electron to primary electron acceptor
3. P680 is reduced by the OEC, which splits an H2O and releases H+ and O2 as products
4. Electron is carried down ETC
5. Creates a proton gradient, which is used to synthesize ATP via P-ATPase
6. Electron excites PSI
7. Gives electron to primary electron complex
8. Reduces NAD+ into NADPH
Describe how the light reactions of photosynthesis make possible the fixation of CO2 by the Calvin cycle.
Light reactions in photosynthesis produce O2, NADPH, and ATP. Both NADH and ATP are reactants For the calvin cycle, which utilizes CO2, NADPH, and ATP to convert a 5-carbon molecule into 2,
3-carbon molecules. One of these molecules can be used for catabolism/anabolism, while the other Is recycled by performing the Calvin cycle 5 times to produce 3, 5-carbon molecules that can be Reused in the Calvin cycle.
Explain the difference between temperature and heat content.
Temperature is the average speed of movement of particles, while heat is the total energy that The substance possesses.
Describe the importance of temperature on physiologic processes, including enzyme stability and kinetics, and the outcome for an organism if body temperature is excessively low or high.
Temperature is important to biochemical and physiological processes, as most enzymes have Optimal temperatures at which the function. If temperature is at an extreme, it can result in Enzymatic failure, as the DNA structure denatures.
Describe the mechanisms by which heat is exchanged, including radiation, conduction, convection, and evaporation, and conditions that favor each exchange mechanism.
Evaporation is a process that requires energy to transform a liquid into a gas, taking energy from the body (HEvap=VTH). Radiation is a process that can either release or intake energy from the environment in the form of infrared electromagnetic waves (Hrad=emissibity SA (T14-T24). Conduction is the process by which heat is transferred via contact (Hcond=Surface Area (T2-T1))/Thickness of insulation. Convection is the process by which energy is transferred via a fluid medium (Hconv = Coefficient SA (T2-T1). Metabolism is the inefficiency within cellular respiration and other biological processes that emits heat as a result.
Describe the sources of heat that contribute to an organism's body temperature, including
endogenous metabolic and exogenous environmental sources, using the heat exchange
equation
TBody=Tambient-Hevap+/-Hconv+/-Hcond+/-Hrad+Hmetabolism. The endogenous metabolic process is metabolism, whereas the exogenous environmental sources include radiation, conduction, convection, and evaporation.
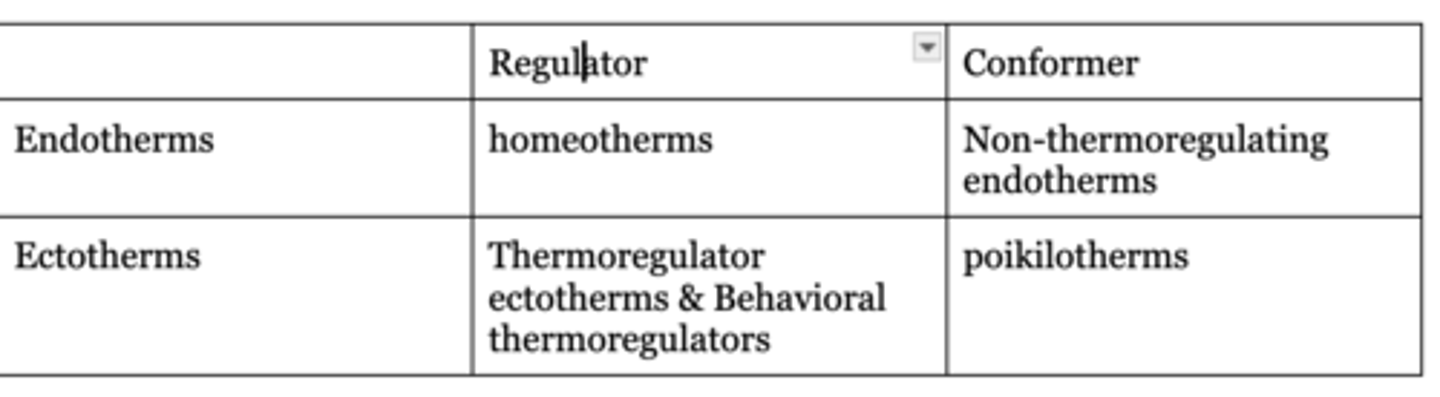
Describe the various categories of organisms (endotherms, ectotherms, heterotherms etc.) in terms of the sources of their body heat, and if and how body heat is regulated.
Include in your description the advantages and disadvantages of strategies used by these categories of organisms.
Endotherms generate a majority of their energy via metabolism, and have high rates of metabolism. Ectotherms rely on the environment for heat, and have low metabolism.
Explain how surface area, volume, and surface area to volume ratios in an organism relate to heat generation and heat exchange.
High surface area allows for large loss of heat, while greater volume means more metabolism. Smaller animals have high metabolic rates per gram. High SA/V ratios signify that more heat Is lost than generated.
Describe how physiologic functions such as metabolic rates, behaviors, and anatomic features influence heat generation heat exchange, and energy conservation. Provide examples with explanations of how they operate.
The pros of endotherms are that they have greater control over their internal temperature and are able to live in many different environments. However, it is energetically draining and requires large bodily complexity.
Higher metabolic rates create higher internal heat, and are a result of fast metabolism. Higher basal metabolic rates produce more internal heat. Behaviors such as seeking out sun, burrowing, or huddling are examples of behavioral regulation that conserve heat.
Identify anatomic features and physiological adaptations of the body that participate in thermoregulation in many animals. Apply the concept of homeostasis to the control of body Temperature.
Regulate via adaption and regulation. Animals diminish surface area and decrease effects of conversion via fur. Countercurrent blood (positioning the two veins close to one another to maintain blood temperature) is another example of adaption. Behavioral adaptions include: pre flight warmup by flies, water byproduct by mosquitos, vasodilation (allows for further heat transfer), and vasoconstriction (decreases heat transfer). Finally, feedback loops—including sweat or shivering—can be used by the body.
Key examples include mosquitoes sweating while feeding, flies warming up before flight, seals developing blubber in cold temperatures.
Finally, brown adipose fat/tissue is used in organisms to produce only heat (does Cellular respiration but does not produce ATP). White fat is used for insulation in humans.