Organic Chemistry Exam 1
1/69
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
70 Terms
Constitutional isomers do not differ in
molecular formula
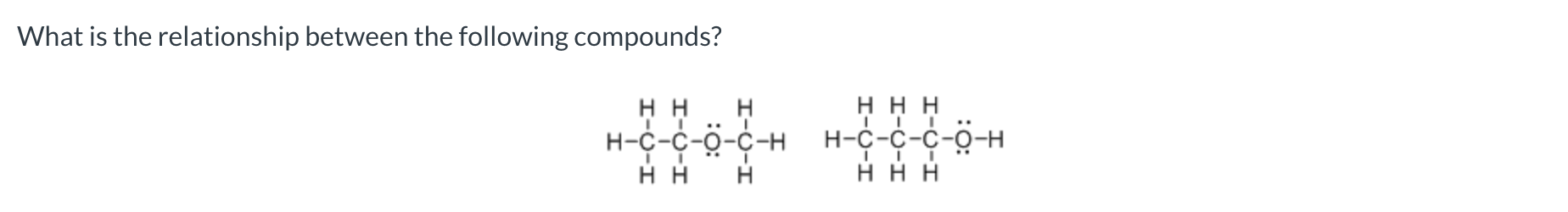
constitutional isomers
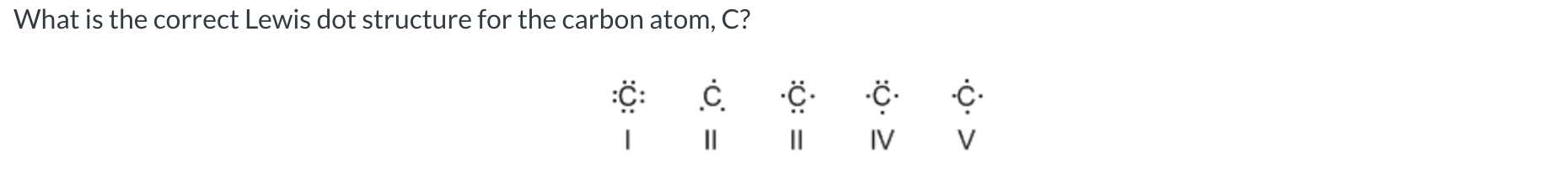
V
I
0
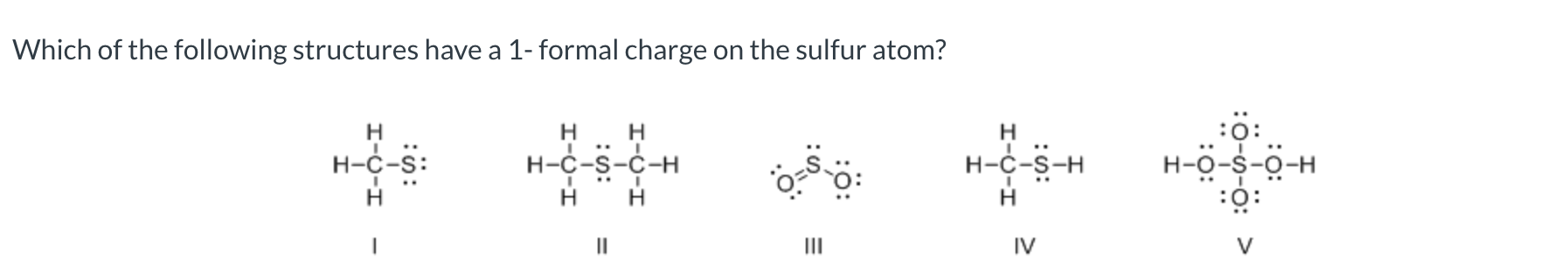
I
Which of the following principles state that “Each orbital can accommodate a maximum of two electrons with opposite spin”?
Pauli Exclusion Principle
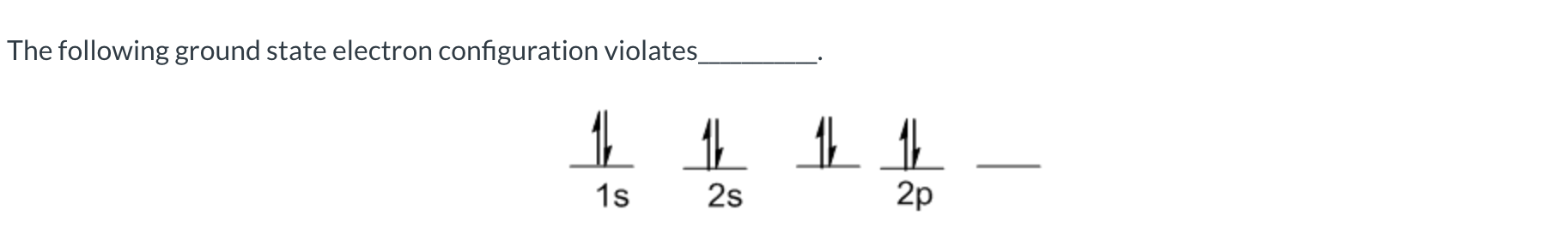
Hund’s Rule
Destructive interference of waves results in
cancellation of both waves and formation of a node
According to molecular orbital theory the highest energy molecular orbital that is occupied with an electron is referred to as
the HOMO
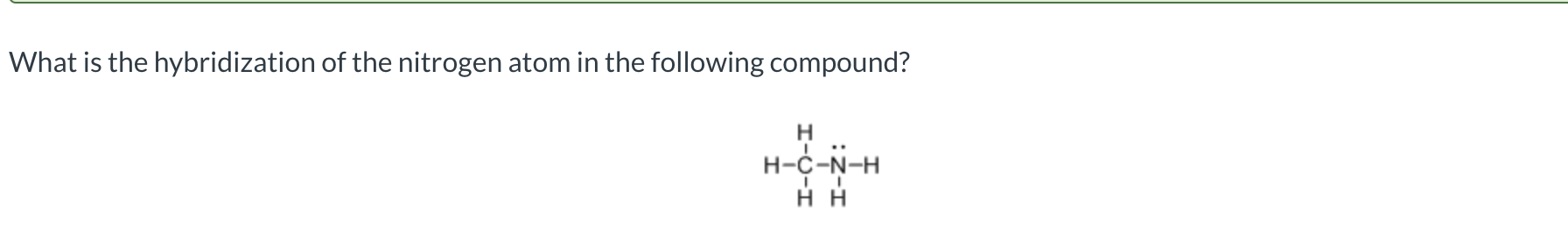
sp³

tetrahedral
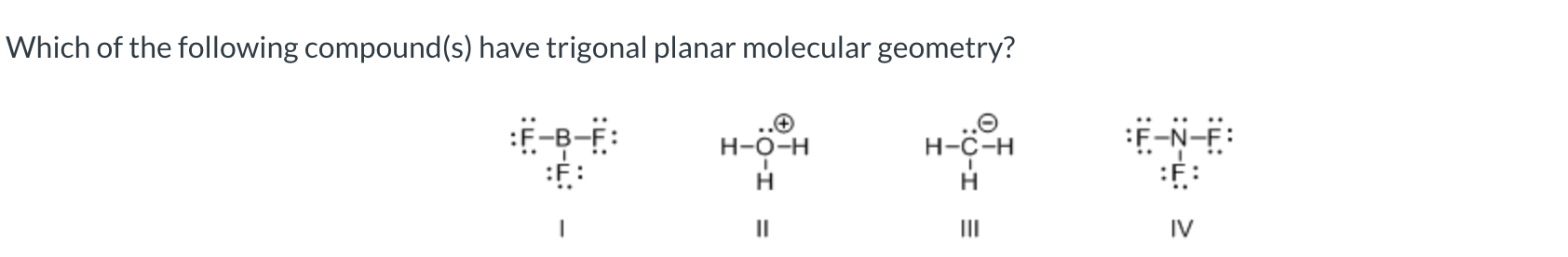
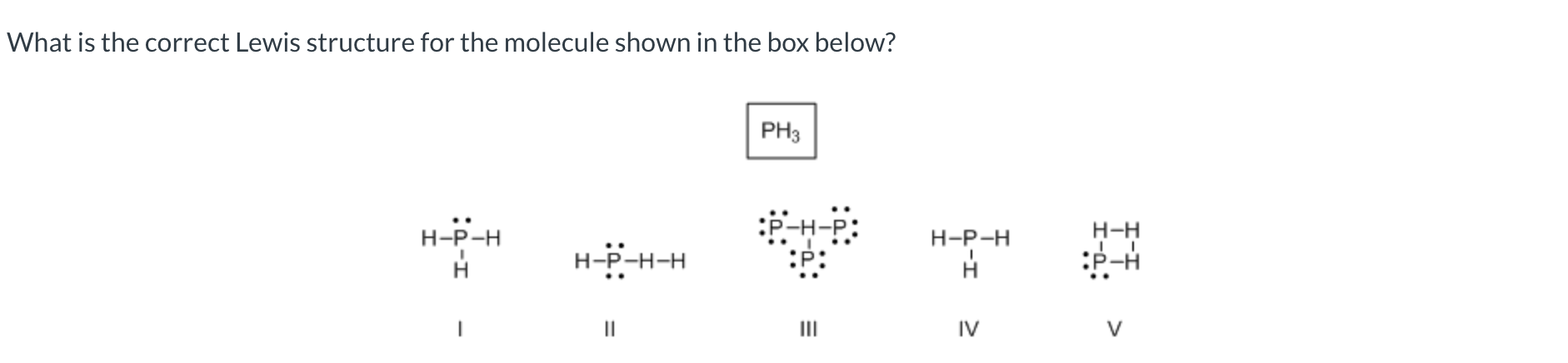
I
Which of the following covalent bonds has the largest dipole moment?
H-F
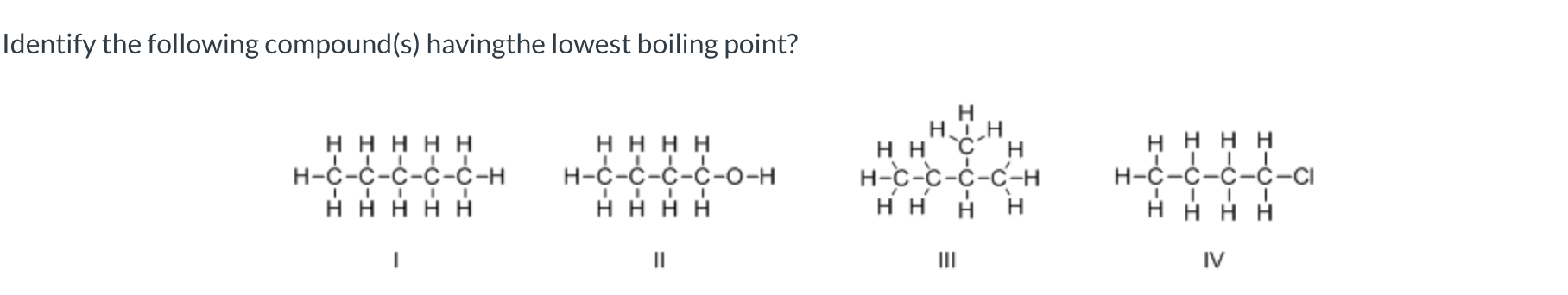
III
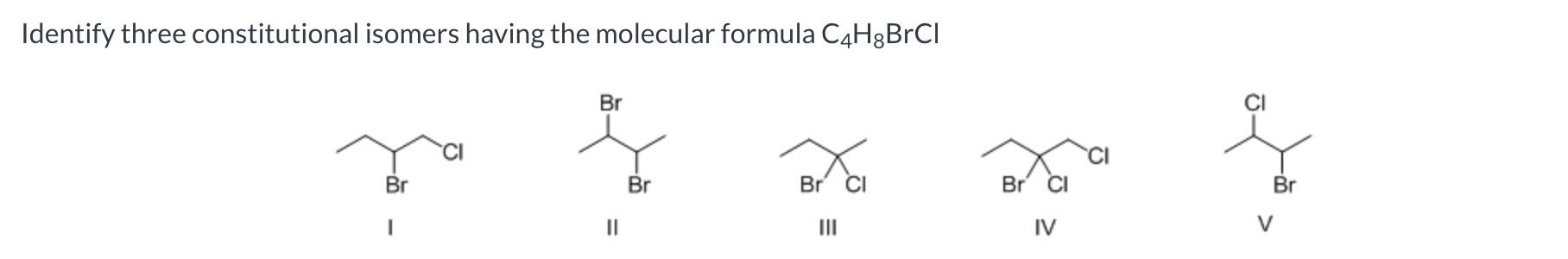
I, III, and V
Which of the following principle states “The lowest energy orbital is filled first”?
Aufbau Principle
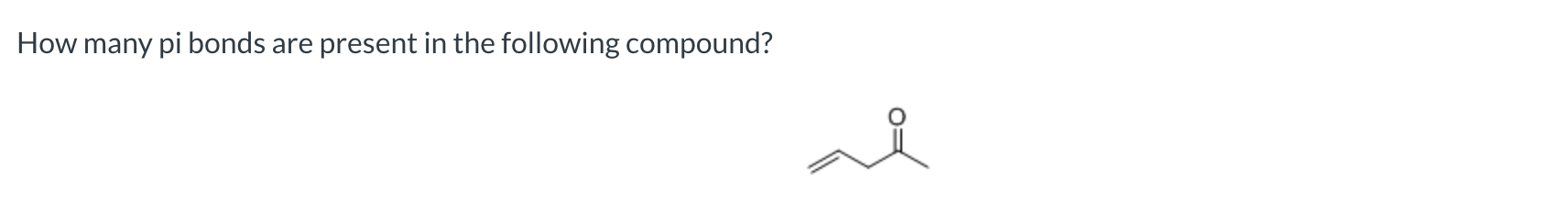
two
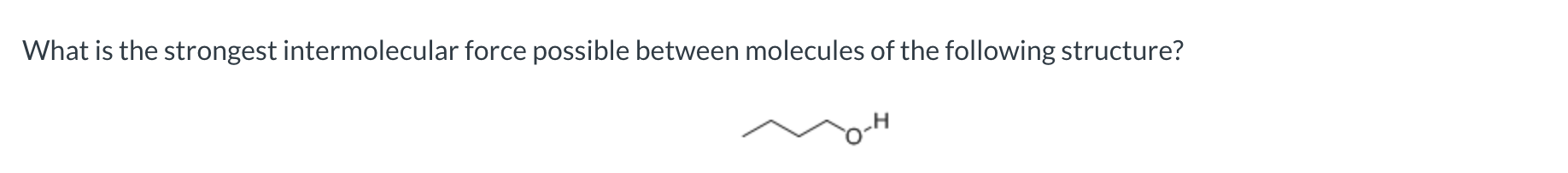
hydrogen bonding
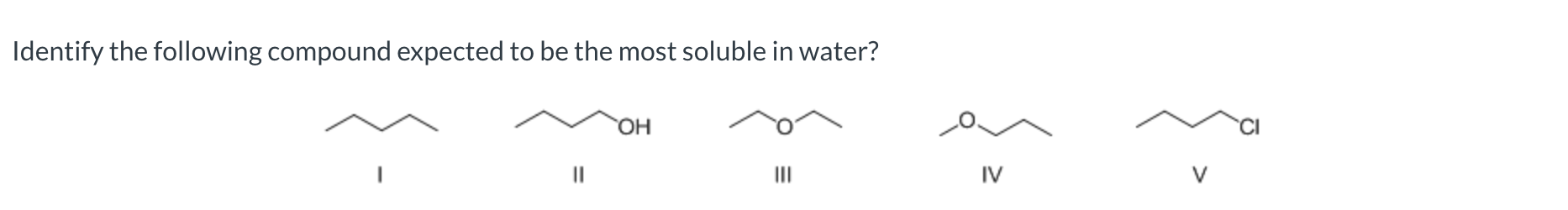
II

1+ on N, 1- on O
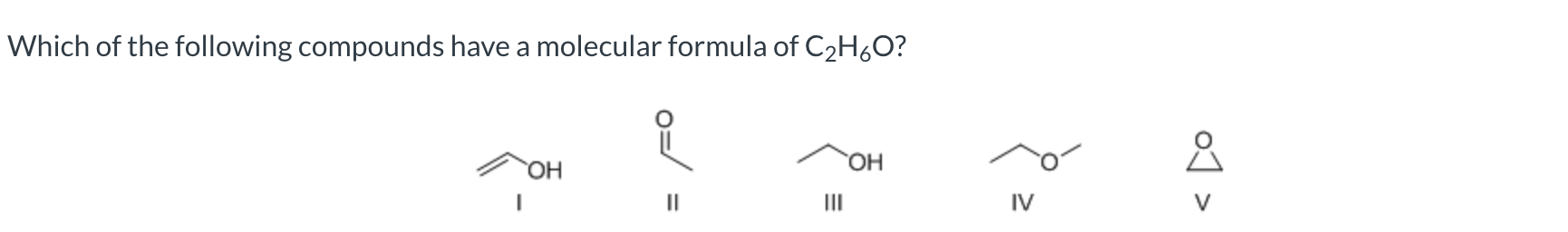
III
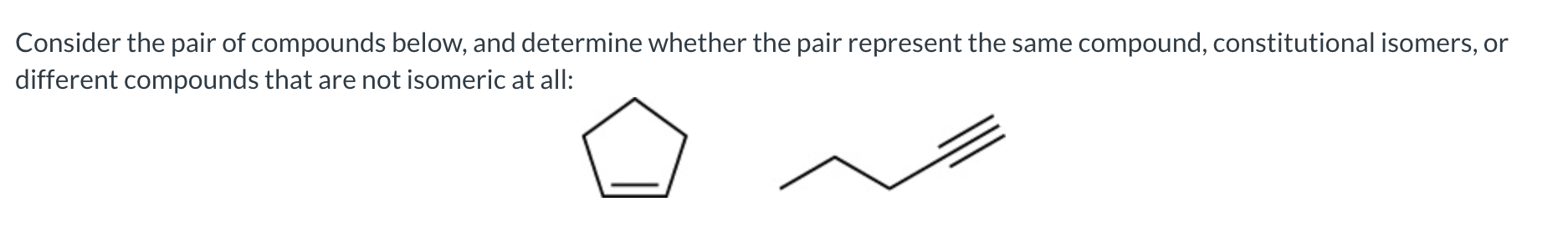
constitutional isomers
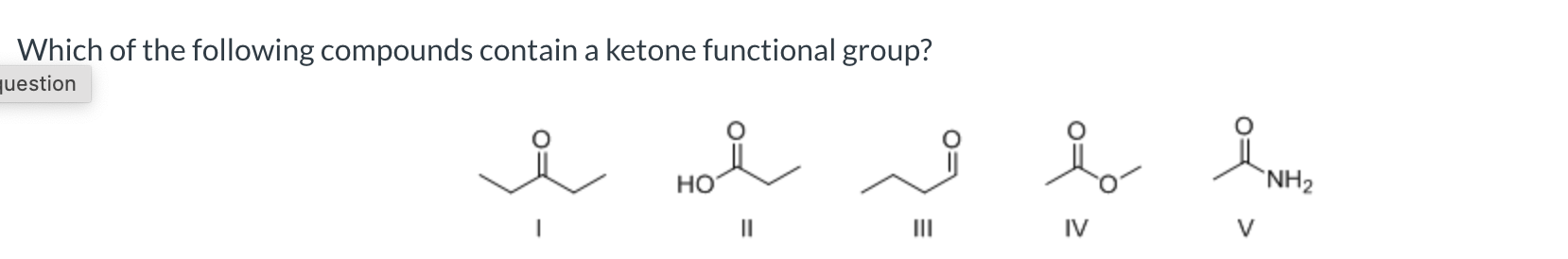
I
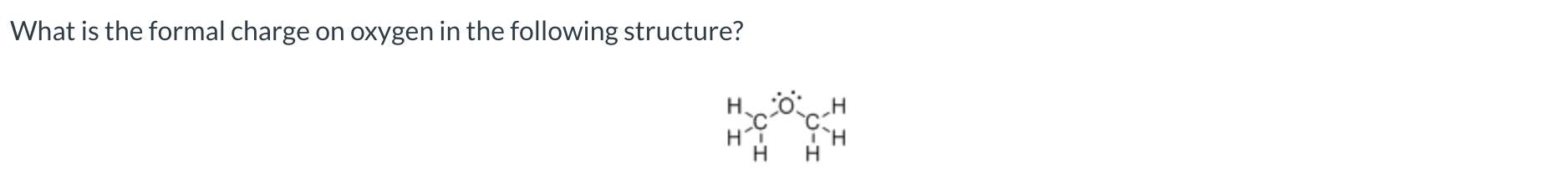
What is the formal charge on a carbon atom with single bonds to three other carbon atoms and one lone pair?
-1

zero hydrogen atoms
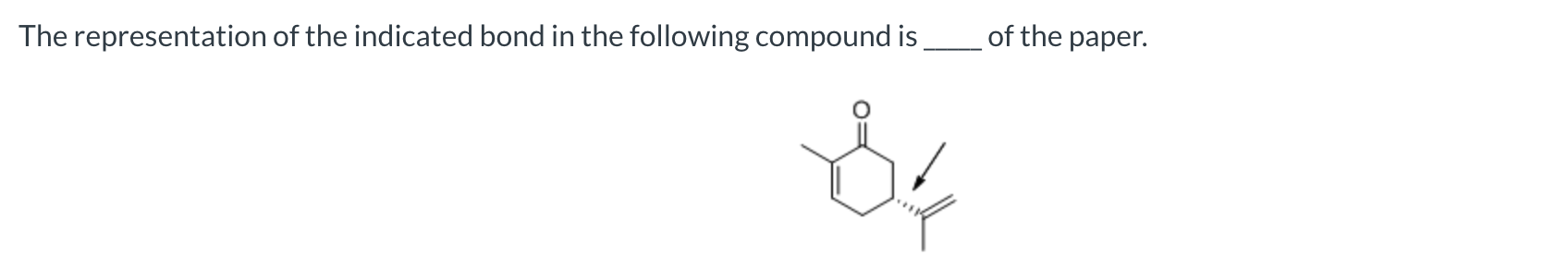
behind the plane
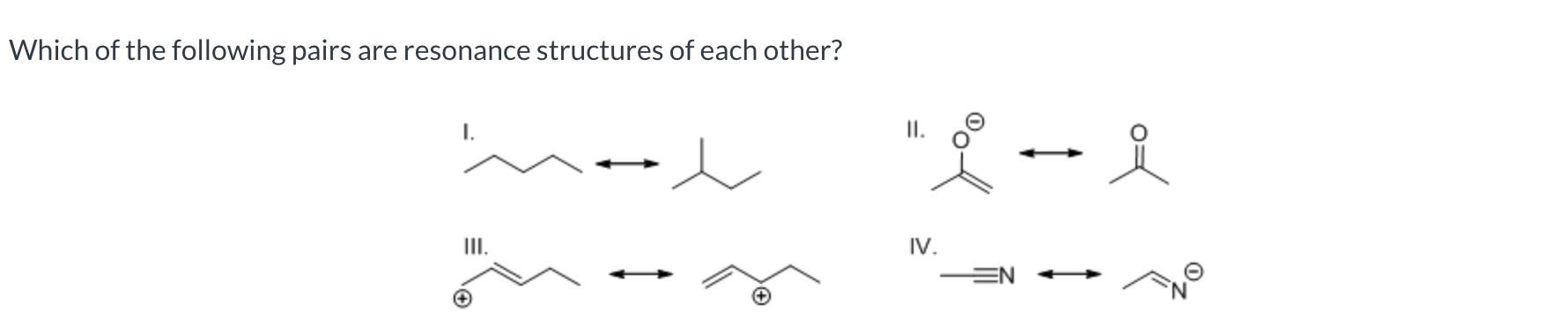
III
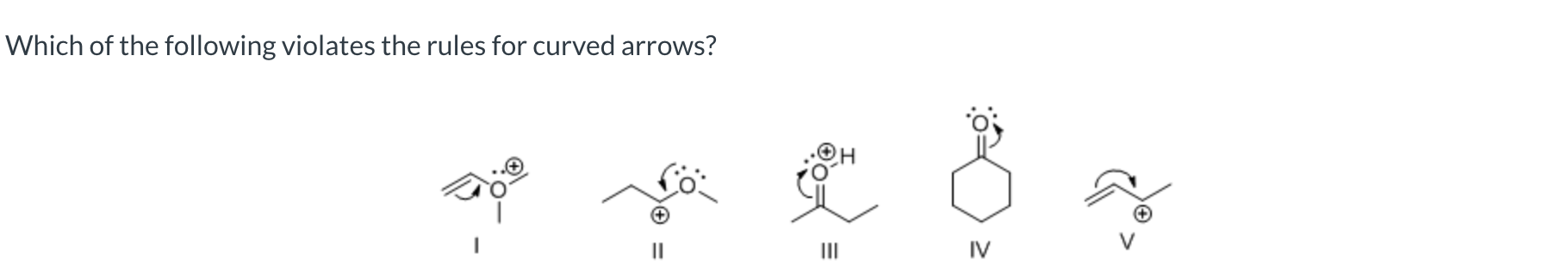
I

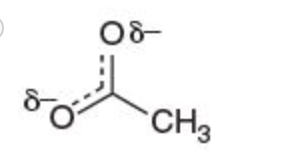
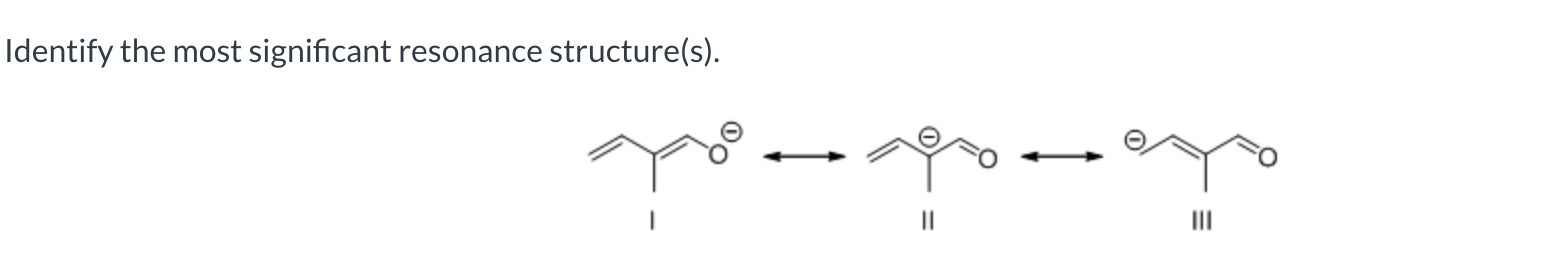
III
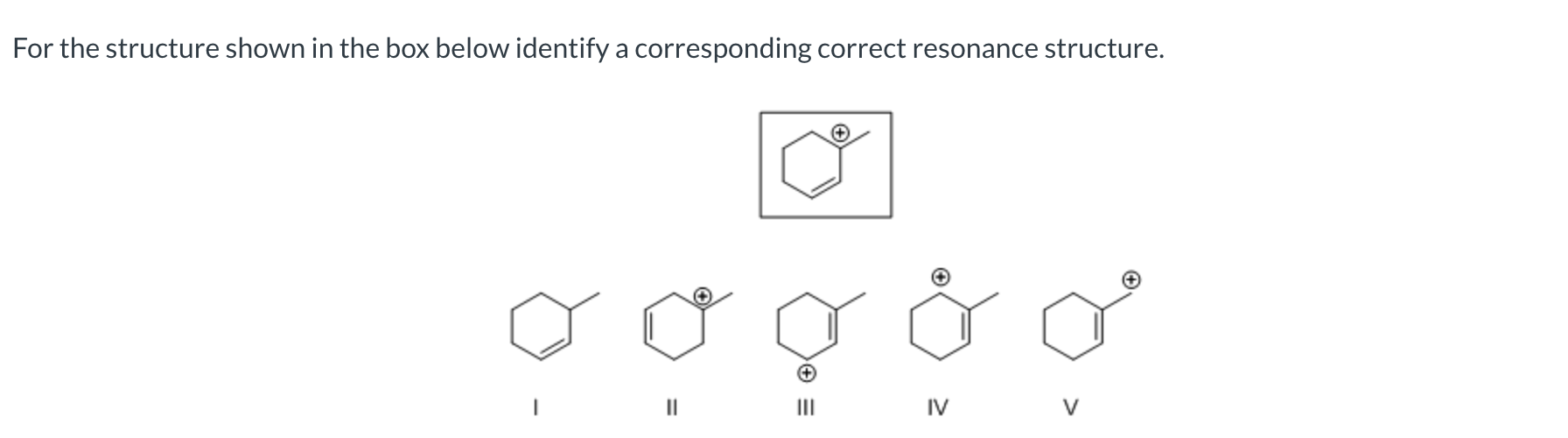
Which of the following has the correct curved arrow(s) placement to show resonance for the given allylic carbocation?
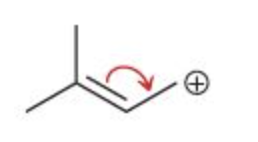
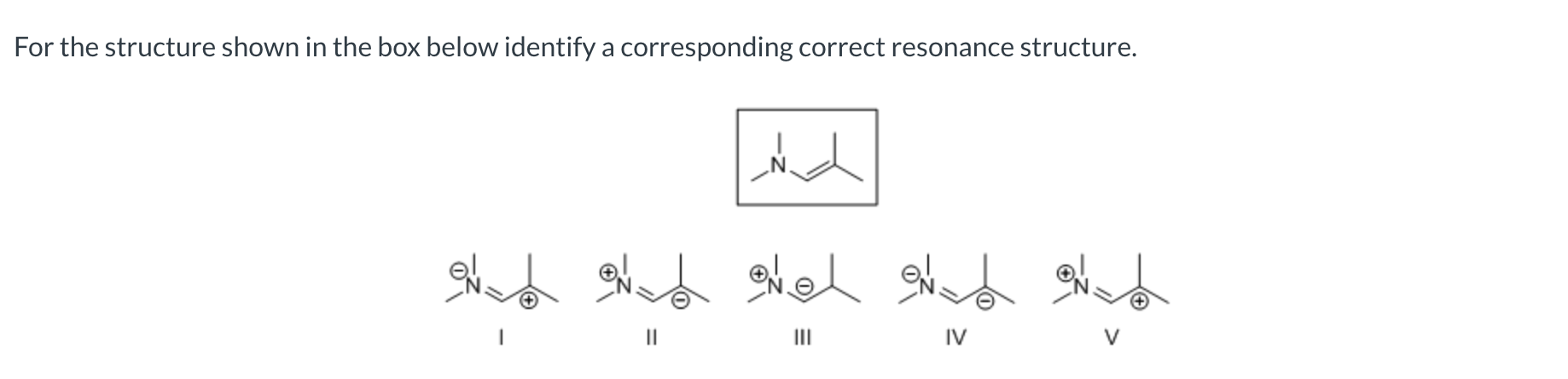
II
I

one localized and one delocalized

a p orbital
Compound A has a pKa of 7 and compound B has a pKa of 10. Compound A is how many times more acidic than compound B?
1000
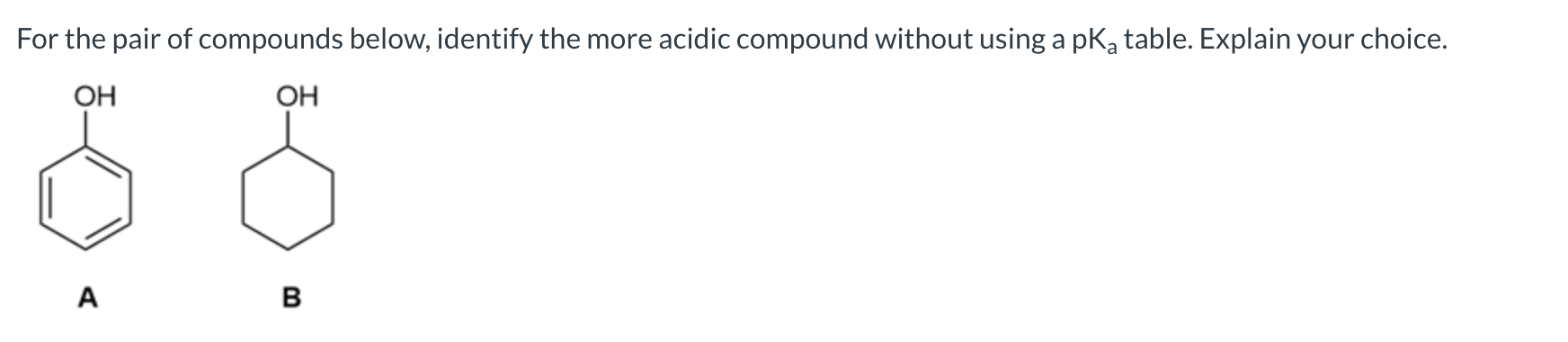
A is the stronger acid because its conjugate base is stabilized by resonance
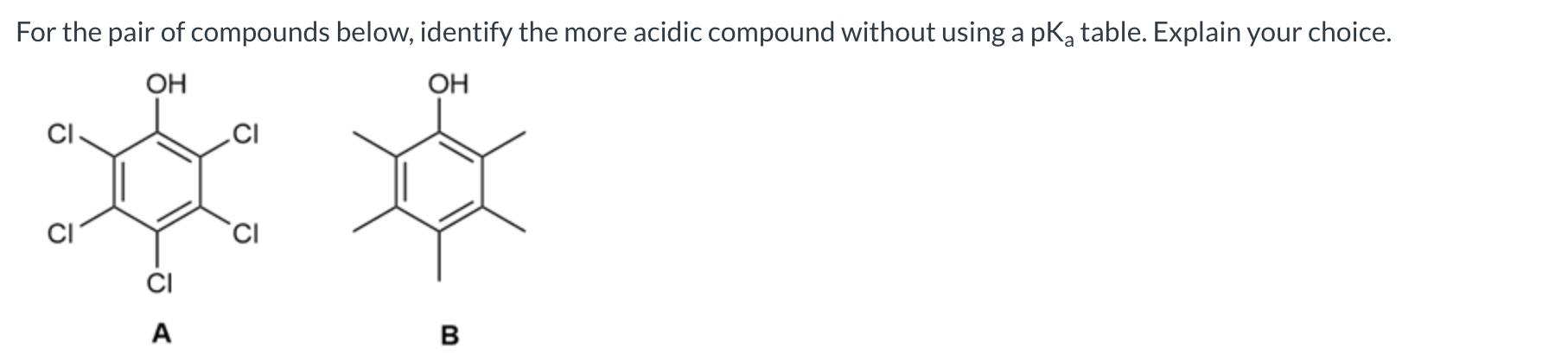
A is the stronger acid because the Cl atoms are electronegative (making the conjugate base of A more stable)

A is the stronger acid because its conjugate base is stabilized by resonance

b
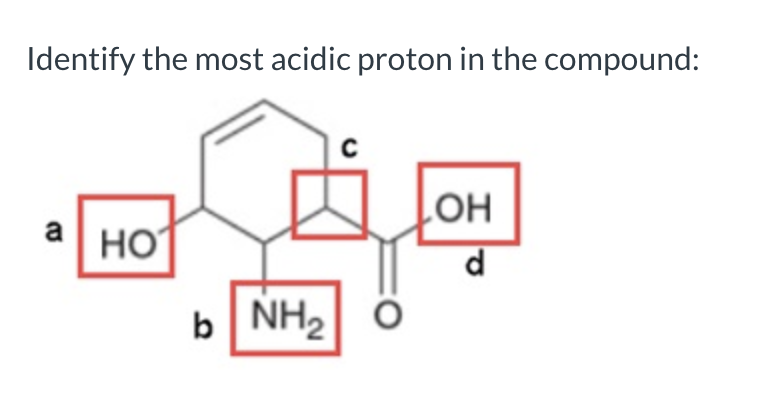
d
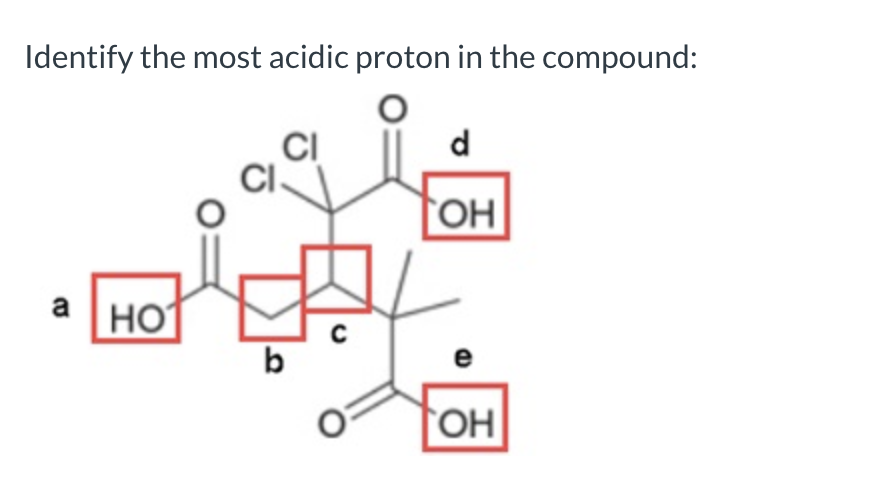
d
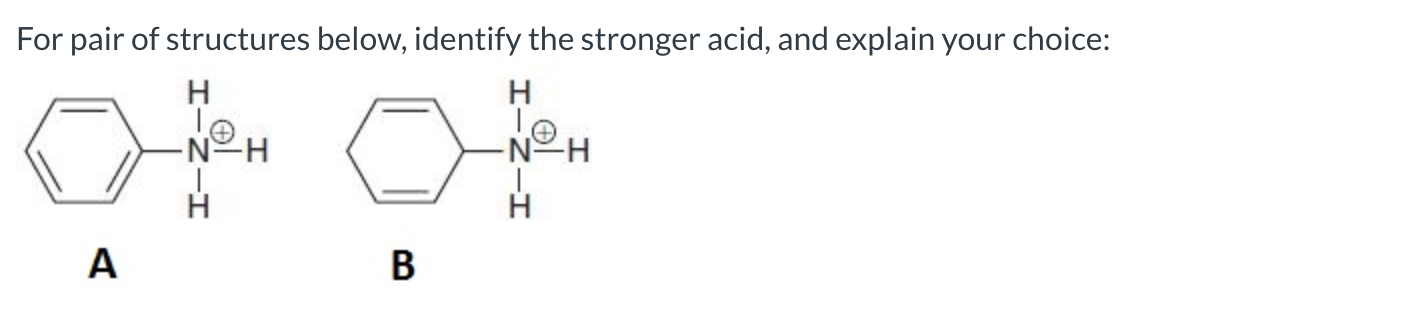
A is the stronger acid because its conjugate base is stabilized by resonance
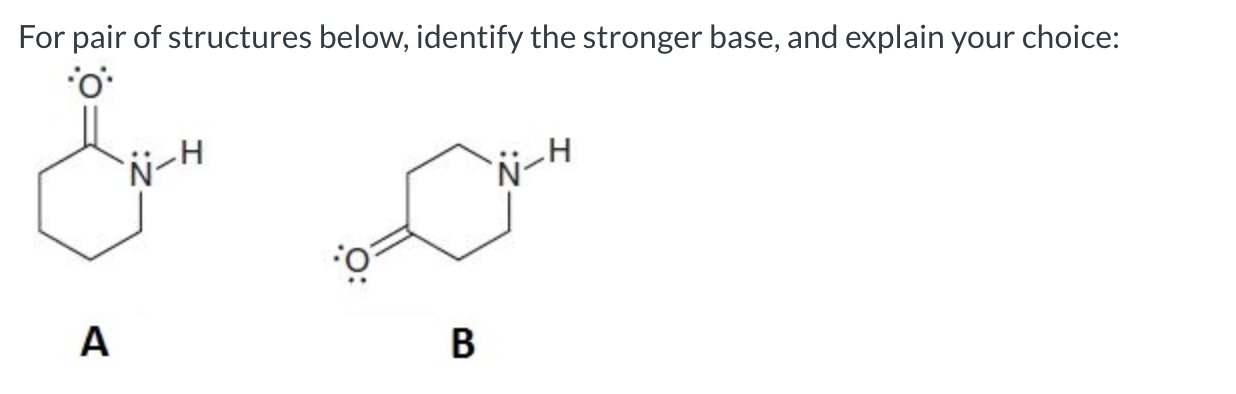
B is the stronger base because A has a delocalized lone pair on nitrogen
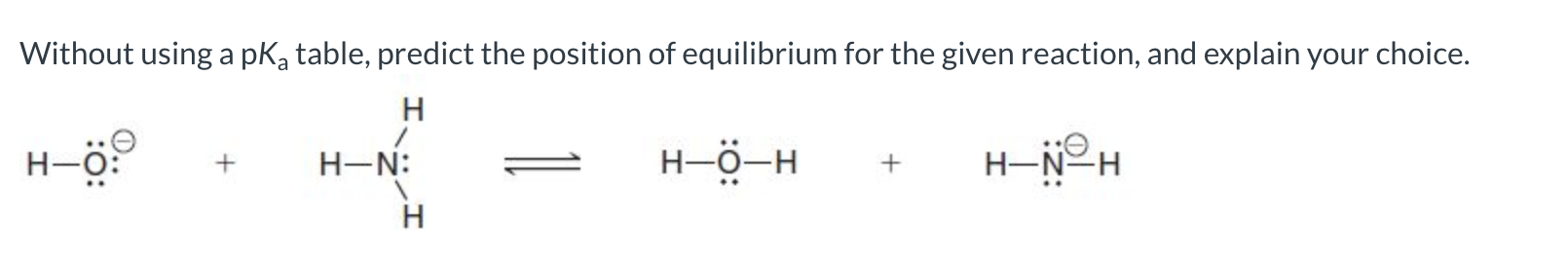
The reverse reaction is favored because oxygen is better at stabilizing the negative charge, making the base on the reactant side the weaker base
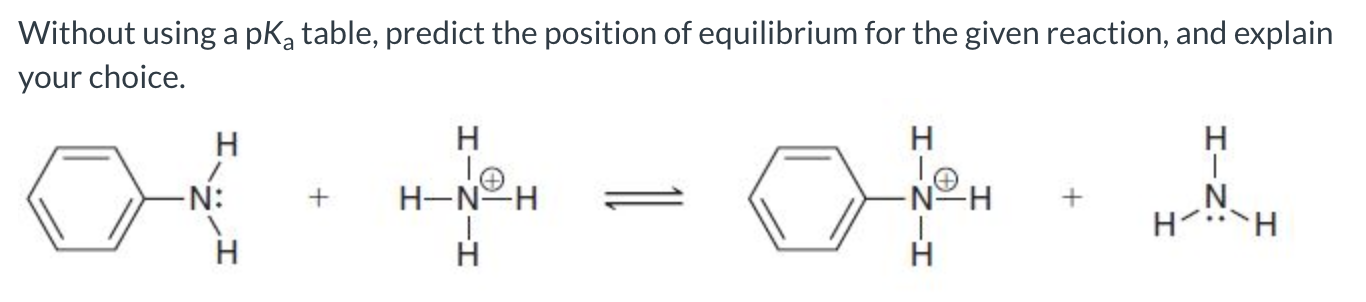
The reverse reaction is favored because the base on the reactant side has a delocalized lone pair, making it the weaker base
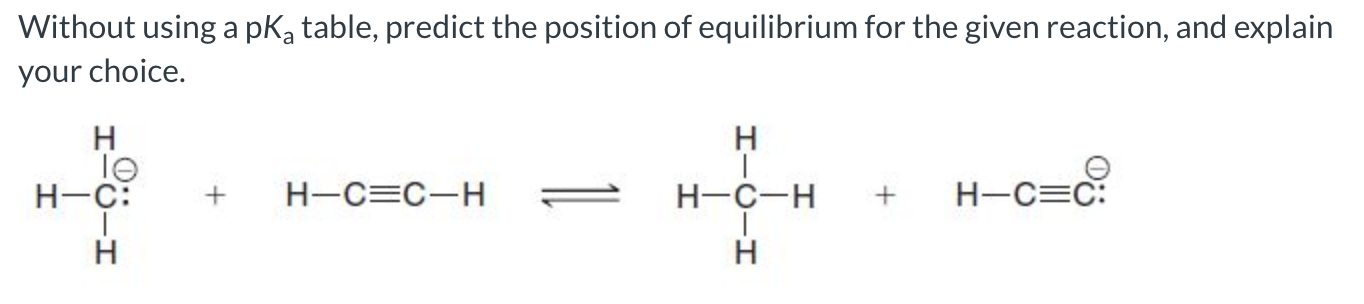
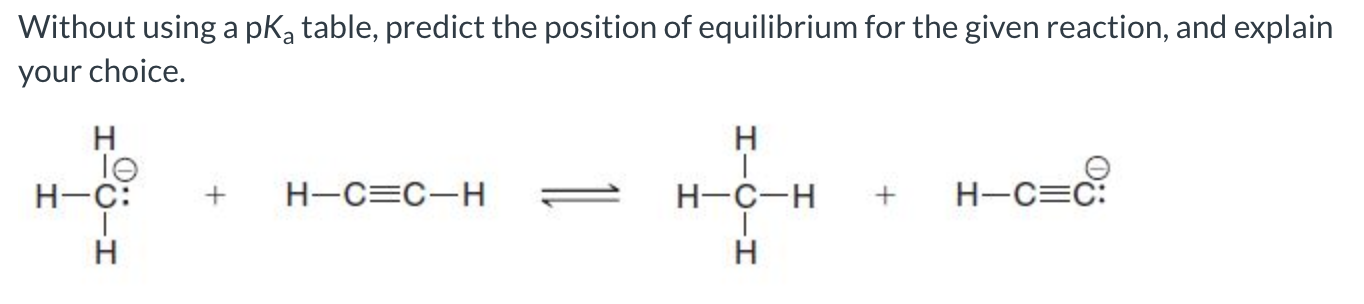
The forward reaction is favored because sp hybridized carbon is better at stabilizing the negative charge, making the base on the product side the weaker base
Identify the correct mechanism for the reaction that occurs between water and a methoxide ion
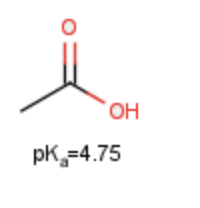
Compare the pKa values of the following two compounds, and identify which is more acidic
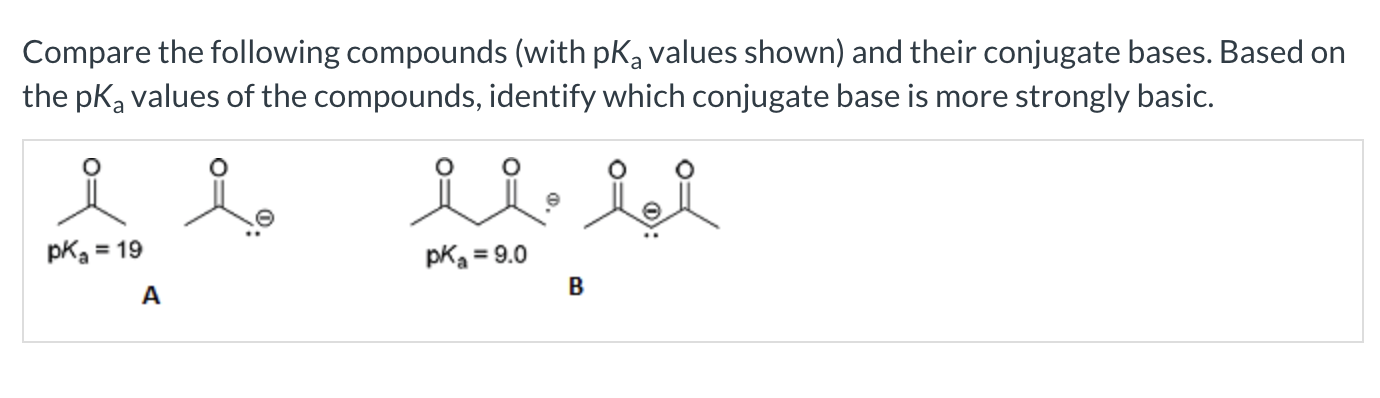
A
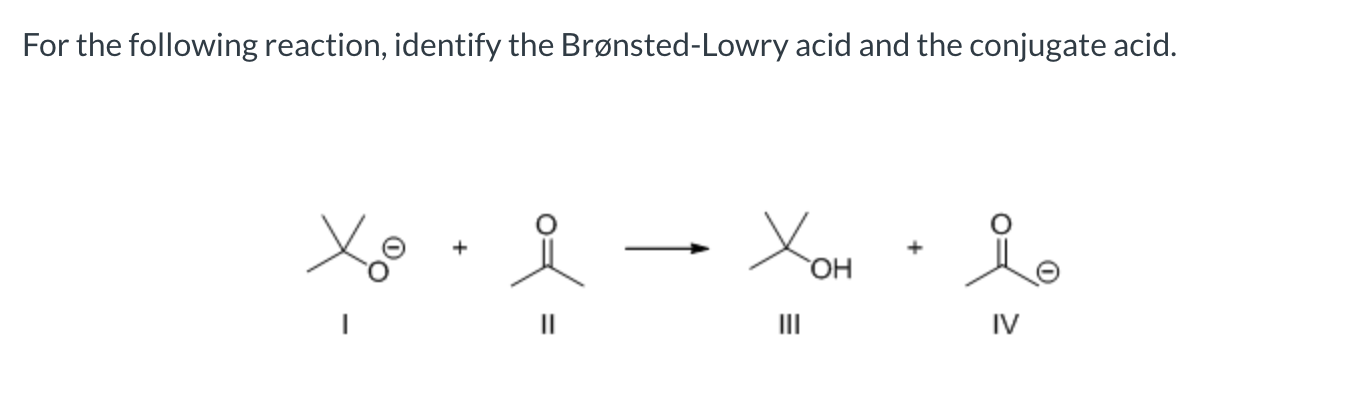
II and III
Identify the conjugate base of CH3CH2SH
CH3CH2-
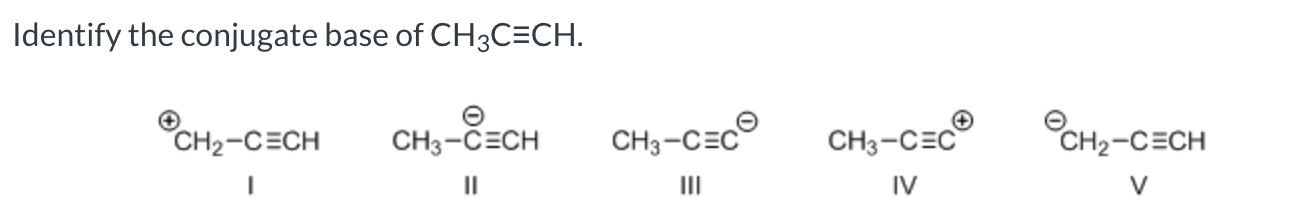
III
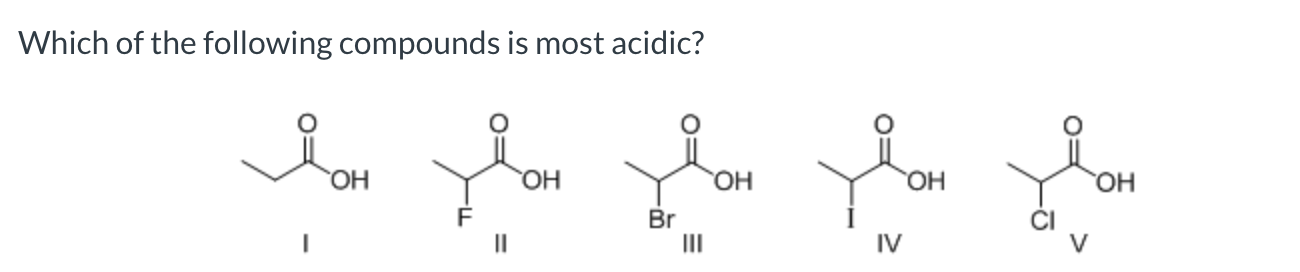
II
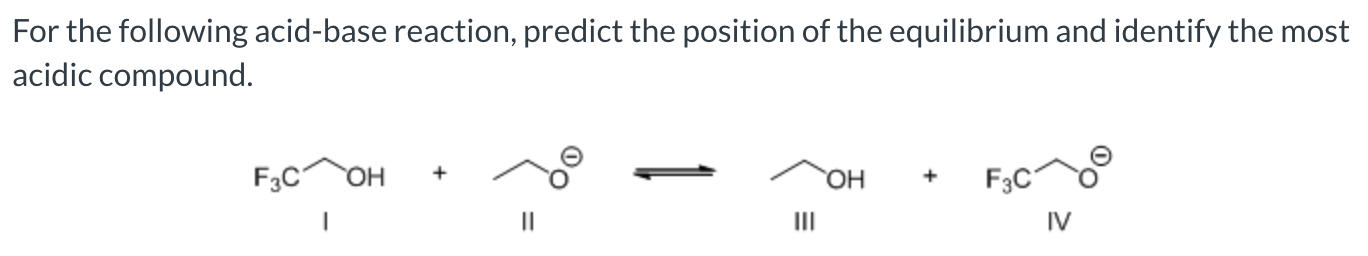
favor the right side with compound 1 being the most acidic compound
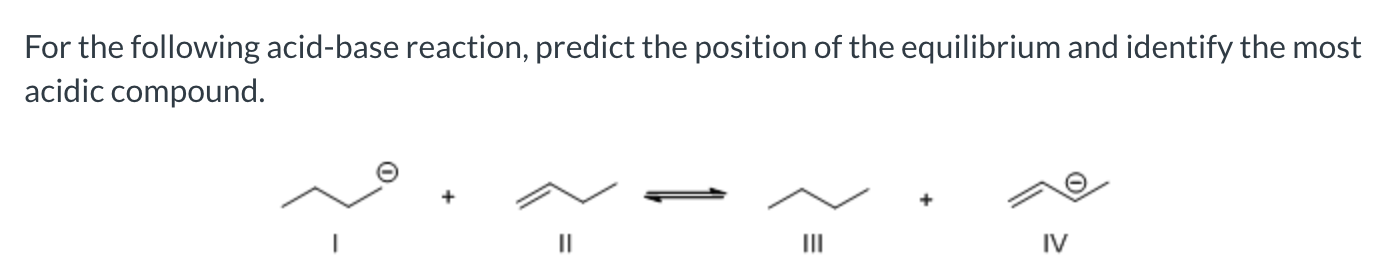
favor the right side with compound II being the most acidic compound
Identify the most sterically hindered alcohol
(CH3)3COH
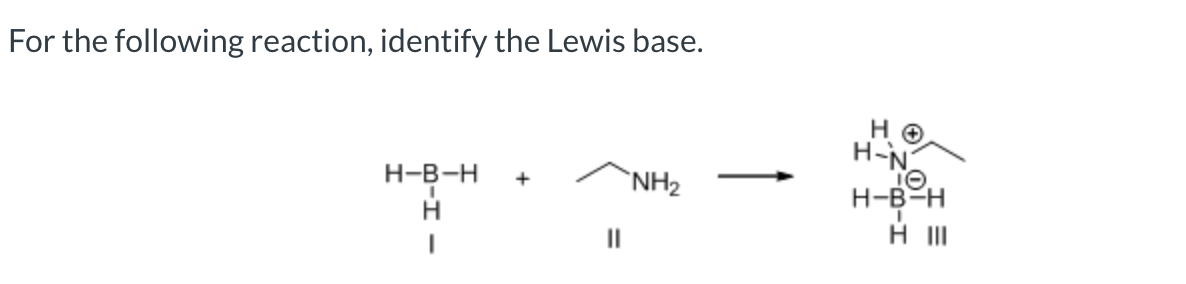
II
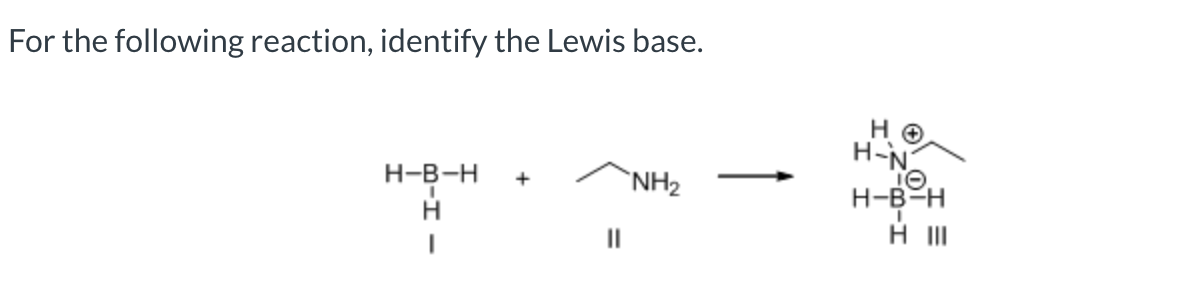
I
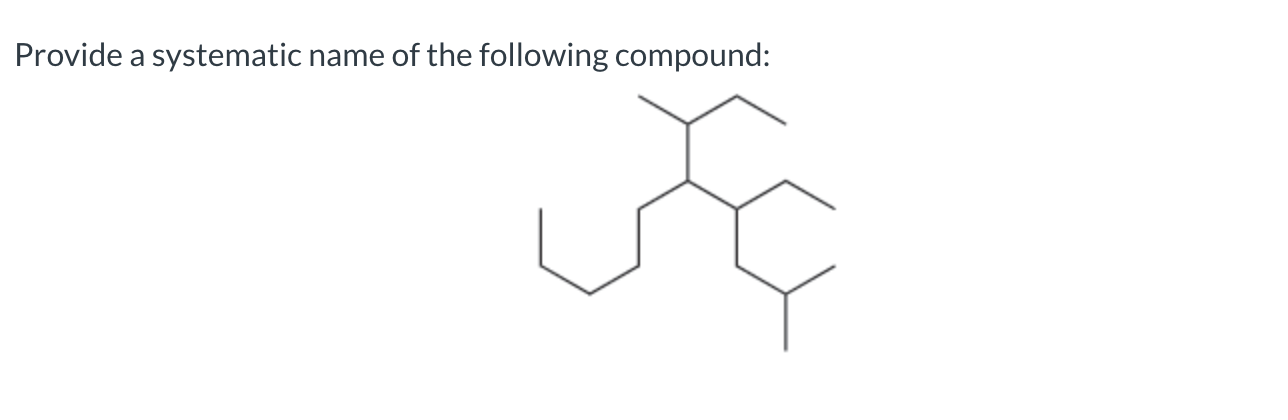
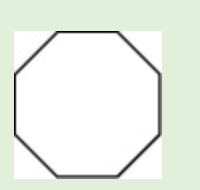
5-sec-butyl-4-ethyl-2-methyldecane
1, 2, 4, 5-tetamethyl-3-propylcyclohexane
Identify which of the following compounds is expected to have the larger heat of combustion
For the following pair of compounds, identify the compound that would have the higher heat of combustion

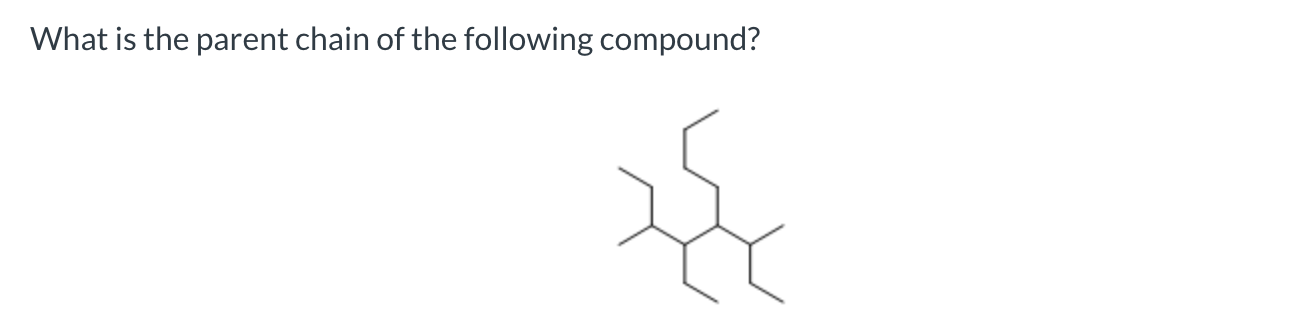
nonane

completely different/not constitutional isomers
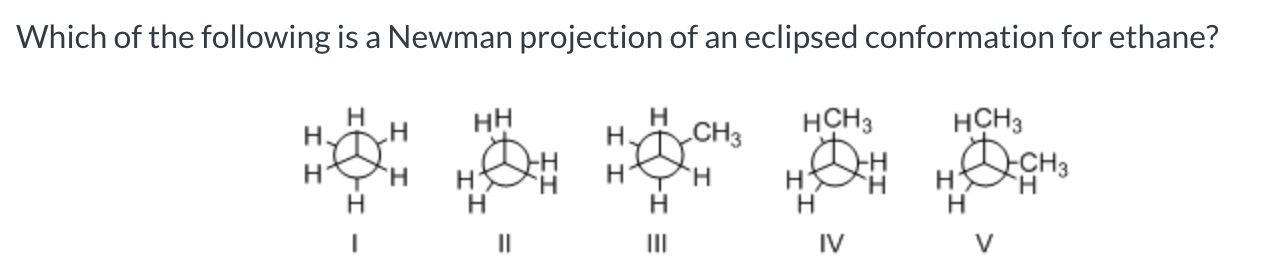
II
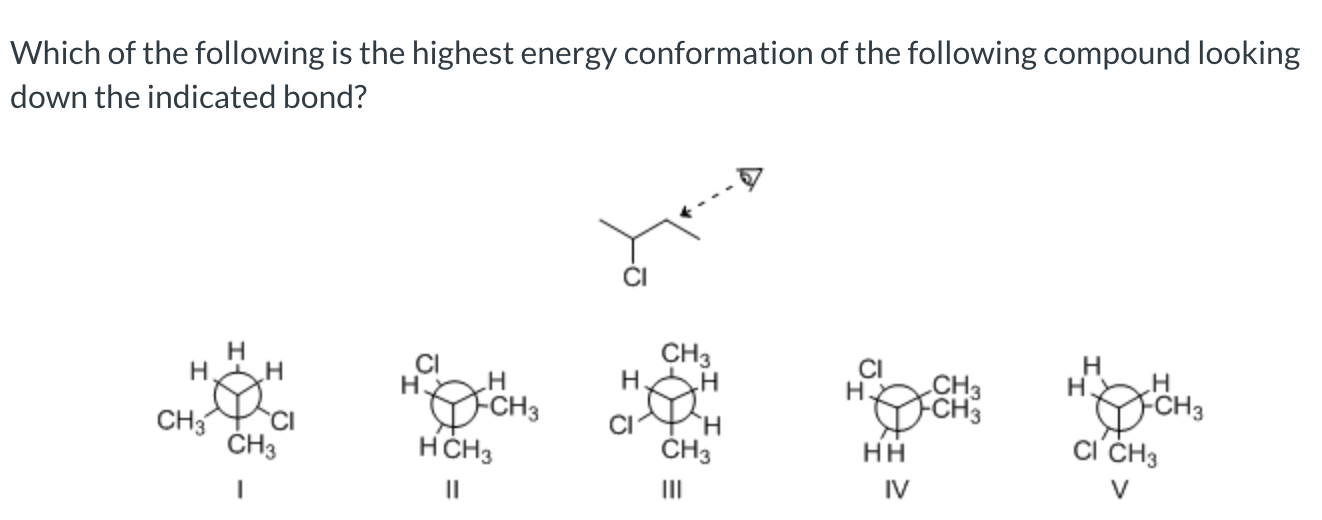
IV
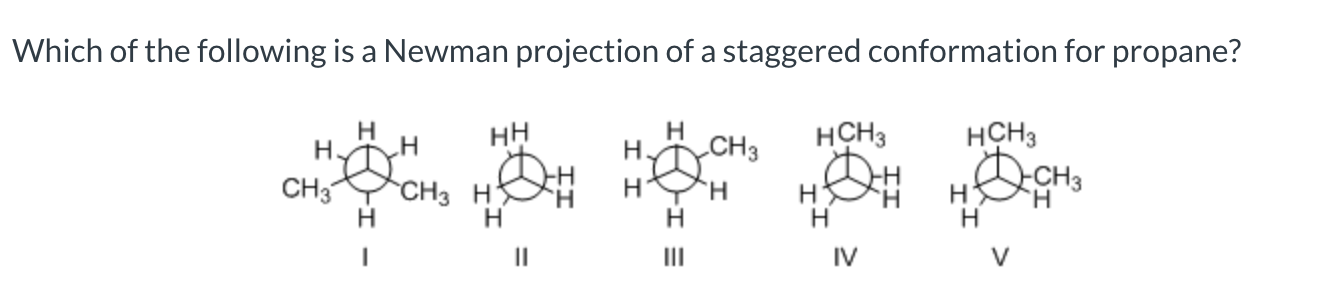
III