BCM.20 - CHEMIOSMOSIS
1/21
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
22 Terms
Peter Mitchell propose....
In 1961 that the ATP made by mitochondria was produced by a chemiosmotic mechanism rather than by substrate level phosphorylation; this was also extremely controversial until the late 1980s.
State the necessary conditions for a chemiosmotic system
1) Proton pump
2) Two compartments ( with a proton impermeable membrane )
3) F-type ATPase
We used to think it was just ...
substrate level phosphorylation directly transferring a phosphate group to ADP.
Evidence for chemiosmosis
- ATP was not made if stalked particles ( ATP synthases ) were removed
- ATP was not made when oligomycin was applied as it blocked the transport of H+ through ATP synthase
- Isolated ATP synthase enzymes can produce ATP using a proton gradient even when there is no electron transport occuring
- pH of intermembrane space is higher than matrix
- When uncouplers were applied the H+ gradient was destroyed and no ATP was made
Example of a non-human chemiosmotic system
Bacteriorhodopsin in Halobacterium
- Purple pigment caused by bacteriorhodopsin which is a trimer and contains retinal ( vit A derived pigment ) which is a green light absorbing pigment.
- When it absorbs the light BR kicks a proton outside cell via flipping a cis bond to a trans bond.
- Conc gradients and voltage gradients are then created.
- Protons flow back through synthases and this turns the F1 component which generated energy that turns ADP +Pi to ATP.
Chemiosmotic systems may...
How?
Predate cellular life
- Lost City alkaline hydrothermal vents
- Bubbly rock akin to cells
- pH gradient across vent's surface minerals: alkaline inside, acidic outside
- Releases warm hydrogen gas that causes precipitation of CaCO3
- Ocean around them is oxidizing. Inside is reducing.
Explain quantitatively how chemiosmosis couples dissipation of a PMF to ATP synthesis
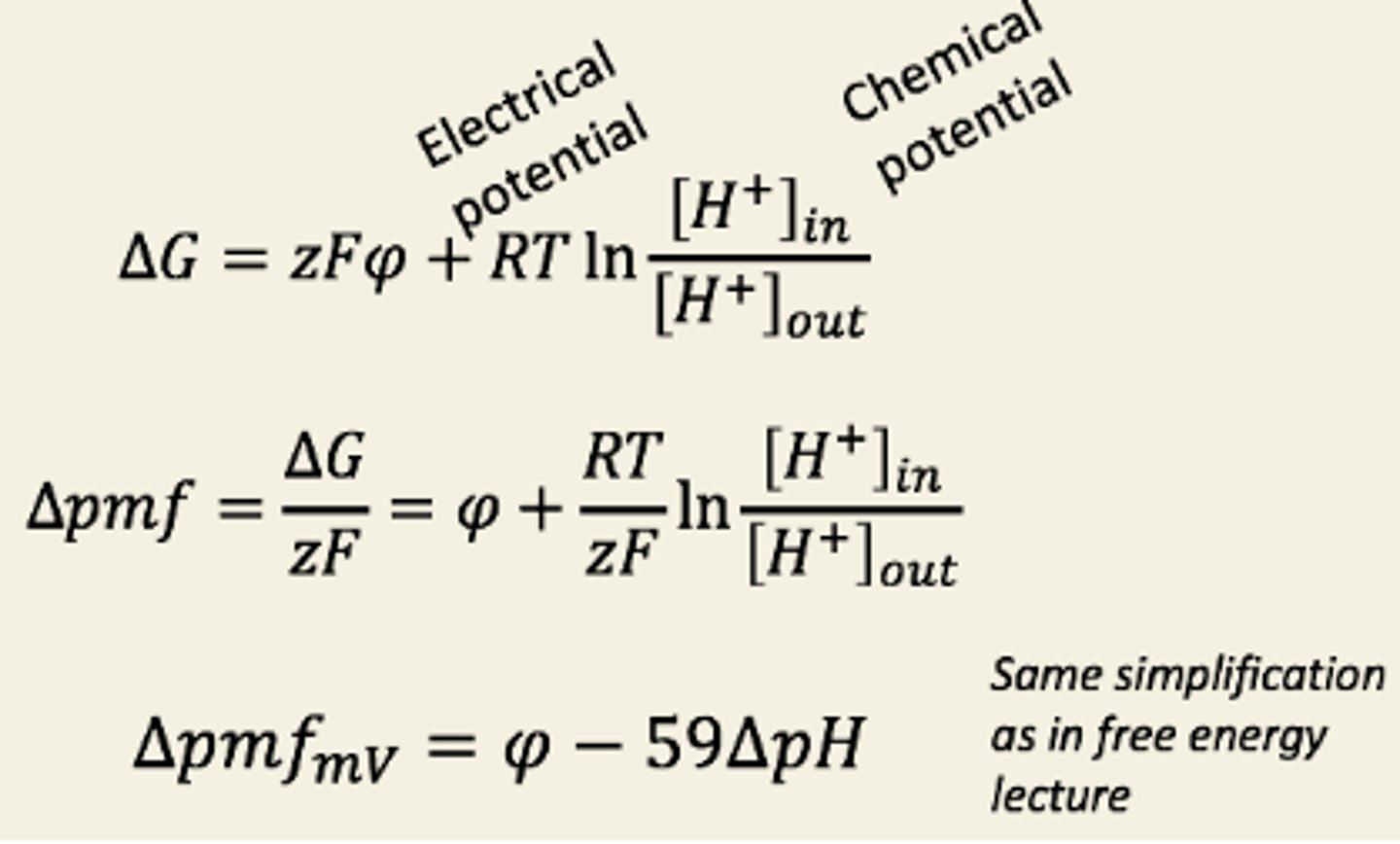
What is the pmf
The proton motive force is the free energy for proton movement, expressed in millivolts.
(energy available to push protons back into matrix)
Proton flow through ...
the FO rotor is coupled to ATP synthesis in F1.
Change in G =
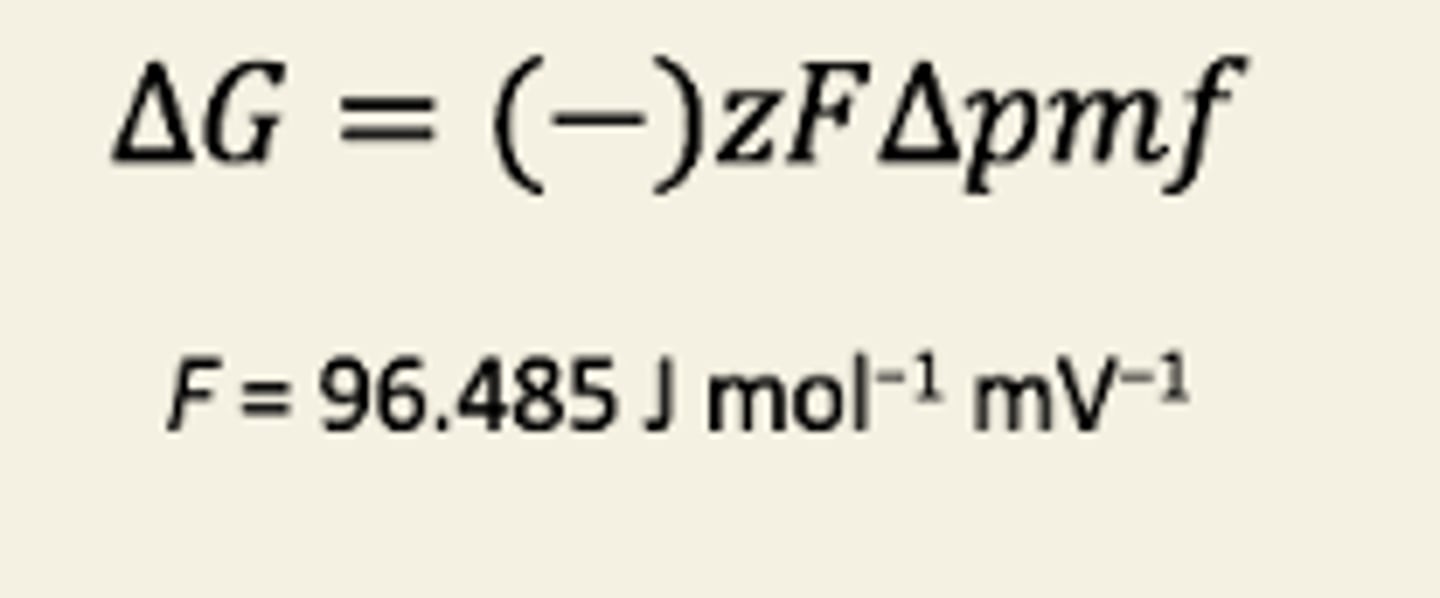
ATP synthase has been shown to ....
rotate during ATP hydrolysis/synthesis when a Fluorescent actin 'tail' was attached to γ of F1.
1 ATP synthase turn =
1 C subunit =
Therefore an octameric c rotor produces how many H+ per turn ?
However
3 ATP
1 Proton
8 H+ / 3 ATP = 2.7 H+ PER ATP
Anything between 8 and 14 c subunits have been seen in different species, and oddly, the 14 is observed in plastids, but these apparently need only 2 protons per ATP!.
Explain the process of chemiosmosis in detail
- Protons flow though the FO portion like a waterwheel: they enter the 'rotor' of c subunits from the 'outside', the whole rotor turns, and they flow out to the 'inside'. The turning of FO is coupled to the rotation of the gamma 'axle', which causes conformational changes in the alpha and beta units of the F1 'headpiece'. The b and delta 'stator' prevent F1 from rotating.
- The β subunits are catalytic, and cycle between 'open', 'loose' and 'tight' binding states. In the open state, free ADP and Pi bind reversibly to the active site. In the loose state, ADP is phosphorylated to ATP. The protein stabilises the ATP, so this step is also freely reversible.
- The loose state is in reversible equilibrium with the tight state, where ATP is bound tightly. It is the release of the ATP from the tightly bound state that requires energy, and this is provided by the motion of the axle, which 'pops' the freshly synthesised ATP from the active site, converting it from a 'tight' state back to an open site which may bind another ADP/Pi.
Why do we often see 4/5 quoted as the proton ATP ratio?
This is because an extra proton is consumed by the translocase that brings in ADP and exports ATP.
We know proton gradients are necessary because ...
Uncoupling agents prevent ATP synthesis:
•Proton channels
•Weak acids
-i.e. proton ionophores
-e.g. Dinitrophenol
How do uncouplers work
Uncouple NADH oxidation ( process driver ) from ATP synthesis. Provides alternative route for dissipation without any energy being used to create ATP.
Energy is turned into heat instead in the cell.
How is PMF generated in short
Proton-motive force is generated by an electron transport chain which acts as a proton pump, using the Gibbs free energy of redox reactions to pump protons (hydrogen ions) out across the membrane, separating the charge across the membrane.
Special case with baby fat
Brown fat mitochondria in babies deliberately uncouple lipid oxidation from ATP synthesis. Baby fat is rich in mitochondria. Purposely do not transfer energy to make ATP but make heat instead due to high SA:VOL ratio as they are likely to get cold faster. Uncoupler is protein channel (thermogenin).
Plants that uncouple on purpose
- Aroids uncouple processes to melt snow to flower earlier / attract flies.
- Arum lilies uncouple their mitochondria to generate heat in their inflorescences. Rather than 'wasting' a proton gradient generated by NADH oxidation, they don't generate such a large PMF in the first place: they use an alternative oxidase that replaces complexes III and IV (i.e. oxidises UQH2 with oxygen), but which does not pump protons. The energy of oxidation is therefore not lost as heat through dissipation of a PMF, but rather more directly: the energy is lost as heat during UQH2 oxidation itself.
Endosymbiotic theory
- 1905-1910 Konstantin Mereschkowski, microbiological evidence by Lynn Margulis in 1967.
- Organelles distinguishing eukaryote cells evolved through symbiosis of individual single-celled prokaryotes (bacteria and archaea).
- Mitochondria, plastids such as chloroplasts, and possibly other organelles of eukaryotic cells represent formerly free-living prokaryotes taken one inside the other in endosymbiosis.
- Mitochondria appear to be related to Rickettsiales proteobacteria, and chloroplasts to nitrogen-fixing filamentous cyanobacteria.
evidence for endosymbiosis
-Transport proteins called porins are found in the outer membranes of mitochondria, chloroplasts and bacterial cell membranes
-Cardiolipin is found only in the inner mitochondrial membrane and bacterial cell membranes
-Some mitochondria and plastids contain single circular DNA molecules similar to the chromosomes of bacteria.
Hydrogen hypothesis
- William F. Martin and Miklós Müller in 1998
1) The host that acquired the mitochondrion was a hydrogen-dependent archaeon, possibly similar in physiology to a modern methanogenic archaea, which use hydrogen and carbon dioxide to produce methane.
2) The future mitochondrion was a facultatively anaerobic eubacterium which produced hydrogen and carbon dioxide as byproducts of anaerobic respiration.
3) A symbiotic relationship between the two started, based on the host's hydrogen dependence (anaerobic syntrophy).