Thermochemistry Review
0.0(0)
Card Sorting
1/4
Earn XP
Description and Tags
Flashcards for Thermochemistry Heat of Reaction Test review.
Last updated 8:53 AM on 5/29/25
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
5 Terms
1
New cards
ΔH (Heat of Reaction) formula
The formula for ΔH (Heat of Reaction) is ΔH = ΣH(products) - ΣH(reactants), representing the change in enthalpy during a chemical reaction.
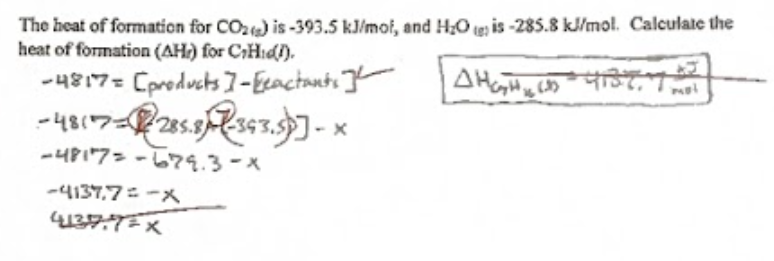
2
New cards
Exothermic Reactions
Reactions that release heat; ΔH is negative with large values.
3
New cards
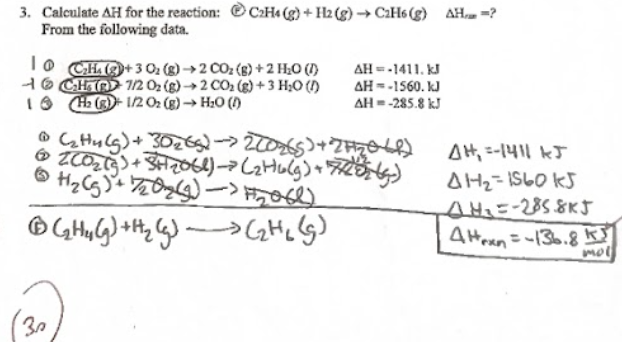
yk how to do these
4
New cards
bruh
bruh
5
New cards
stoichiometry
