Organic Chemistry Spectroscopy
1/24
Earn XP
Description and Tags
Chpt 12, 13,
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
25 Terms
What information does Infrared Spectroscopy give?
Functional groups
What information does Mass Spectroscopy give?
Molecular weight & formula
IR Spectroscopy, what appears around 1600?
Alkenes
Aromatic ring
What shows up around 1600-2000 (IR)?
double bonds
What shows up around 2000-2500 (IR)?
triple bonds
What shows up around 2600-3000 (IR)?
hydrogen bonds
What functional group is present around 3200-3500 and shows a strong peak (IR)?
Alcohol
What functional group presents around 1700-1725 (IR)?
Carbonyl group
What shows around 2700 (IR)?
Aldehyde C-H stretch
Which functional group gives us nothing (IR)?
Ethers
Which functional group presents two peaks around 1300-1000 (IR)?
Ester C-O stretch
An electronegative hydrogen bond shows around 3200-3000 with a weak peak (IR), which atom is it?
Nitrogen
Alkenes peaks are …?
weak
What is the molecular ion?
mw of original compound
Where does the molecular weight show up? It’s the ______ in its group.
Far right
Tallest
What is the base peak?
Tallest peak
What does m+2 give?
halides present
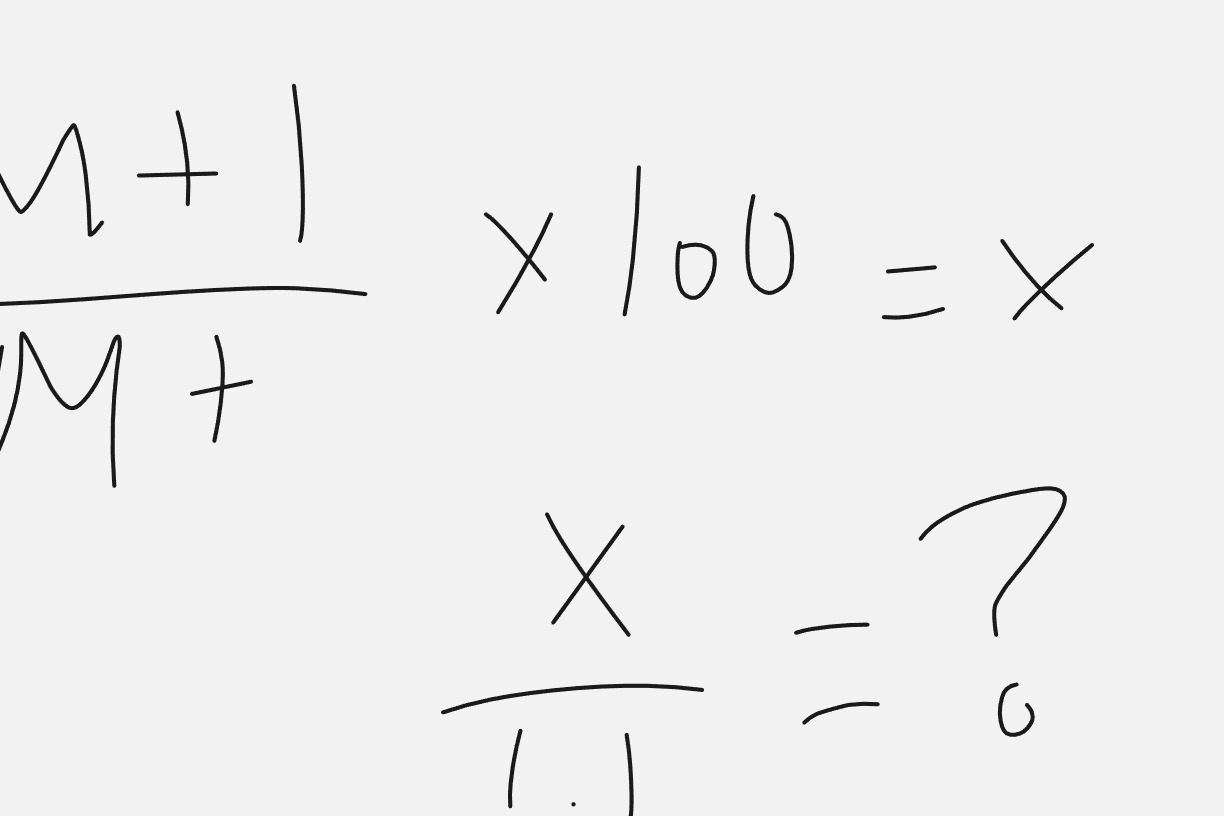
What is this formula calculating?
# of carbons
If M+1 is absent, how can the number of carbons be found?
Divide M+ by 12
What is the ratio of Br (MS)?
1:1
What is the ratio of Cl (MS)?
3:1
What is the ratio of sulfur (MS)?
100:4
What information can the m/z tell us?
mw
# of Ns
Which atom could be present but does not show on the MS?
O
What mass should be used to calculate the amount of each type of atom?
Nominal