Electron transfer reactions
1/31
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
32 Terms
oxidation
the loss of electrons from a substance
or the gain of oxygen
reduction
the gain of electrons
or the loss of oxygen
oxidising agent
oxidises another substance by causing it to lose electrons, but gets reduced itself
oxidation number of oxidising agent decreases
change in oxidation number is equal to the number of electrons involved in the half equation
reducing agent
reduces another substance by causing it to gain electrons, but gets oxidised itself
oxidation number of reducing agent increases
change in oxidation number is equal to the number of electrons involved in the half equation
how to balance a redox equation
write the unbalanced equation and identify which atoms change in oxidation number
deduce oxidation number changes
balance the oxidation number changes
balance the charges
balance the atoms
redox titrations
an oxidising agent is titrated against a reducing agent
most transition metal ions naturally change colour when changing oxidation state, so indicators rarely needed
ease of oxidation
relative ease of oxidation increases down the group for group 1 & 2 metals
reactions become more vigorous
reduction of halogens
halogens are oxidising agents, as they remove an electron from the metal they react with, and themselves gain an extra electron from the metal
oxidising power of halogens decreases going down the group
they become less reactive
metal reactivity series
metals in higher reactivity can displace less reactive metals from their compounds in solutions / oxides
the more reactive metal acts as a reducing agent

acids with reactive metals
acid + metal —> salt + hydrogen gas
extent of reaction depends on reactivity of metal and strength of acid
reactions of acids & metals can be written as ionic equations showing only species that has changed in reaction
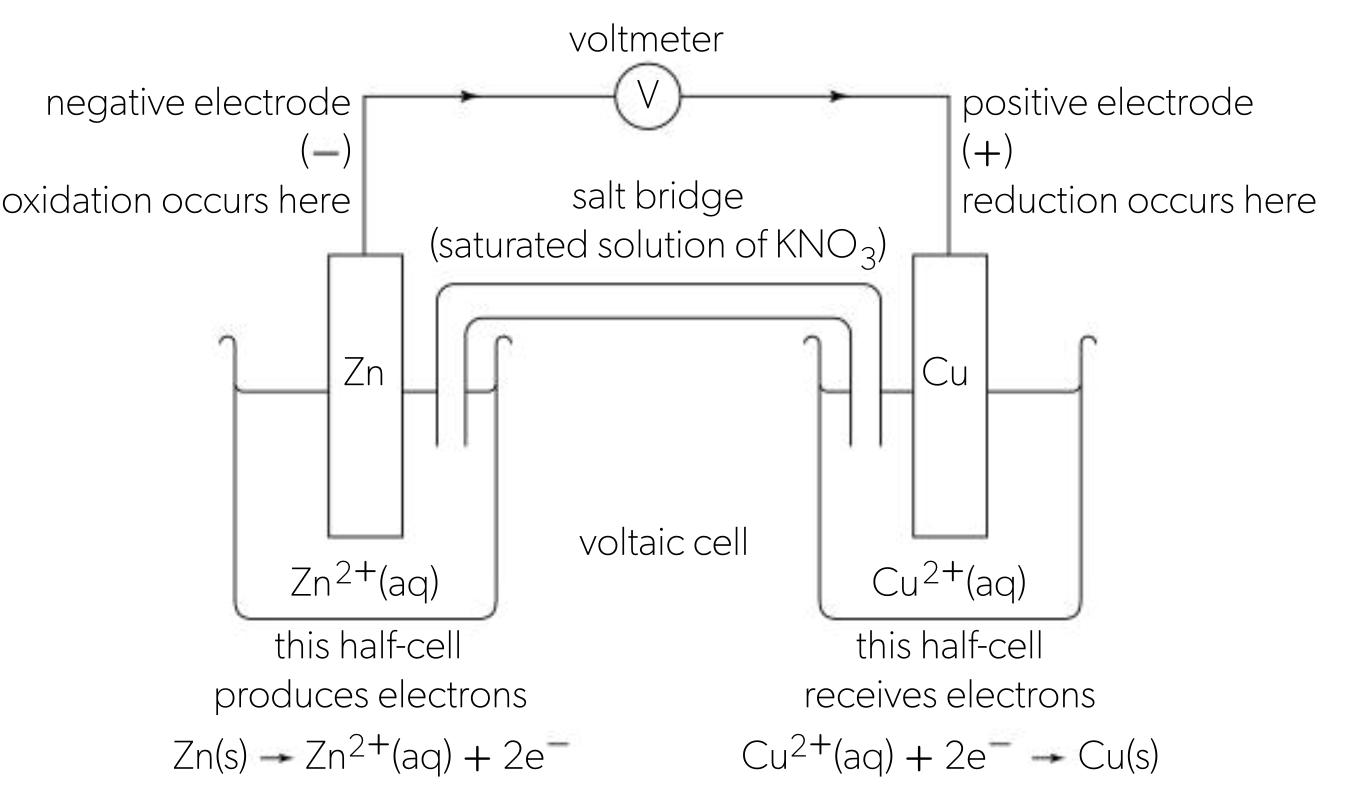
primary (voltaic) cells
convert energy from spontaneous redox reactions to electrical energy
generate a potential difference known as EMF (E), also called cell potential
a half cell is a metal in contact with an aqueous solution of its own ions, a primary cell consists of 2 different half cells
2 half cells are connected together to enable transfer of electrons to produce energy as electricity
cells are connected by an external wire and salt bridge
salt bridge allows free movement of ions
primary cell example
zinc metal strip (electrode) dipped into zinc sulphate connected to a copper electrode dipped into copper sulphate
zinc is more reactive, so electrons flow from the zinc half cell towards the copper half cell
to keep half cells electrically neutral, ions flow through the salt bridge
negative ions flow to the negative half cell (Zn)
positive ions flow to the positive half cell (Cu)
voltage produced by a voltaic cell depends on the relative difference between the 2 metals in the reactivity series
bigger diff = higher voltage produced
fuel cells: primary
electrochemical cell where a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode
as the fuel enters cell, it becomes oxidised, setting up a voltage within the cell
e.g hydrogen-oxygen fuel cell
advantages:
water is the only reaction product, so better for environment than other cells
no harmful oxides of nitrogen produced
disadvantages:
hydrogen is highly flammable and its storage carries safety hazard
very thick pipes needed to store hydrogen, has economic impacts
convention for writing cells
the half cell undergoing oxidation is placed on the left and half cell undergoing reduction is on right
shorthand notation:
half cell is denoted by metal/metal ions and II to denote the salt bridge
e.g Zn/Zn2+II Cu2+/Cu
secondary (rechargeable) cells
employ chemical reactions which can be reversed by applying a voltage greater than the cell voltage, causing electrons to push in the opposite direction
examples:
Lead-acid battery
nickel-cadmium cell
lithium cell
lead-acid battery: secondary
6 cells joined together in series, produce total voltage 12 V, used in cars
lead metal as negative electrode, lead (IV) oxide as positive electrode, sulphuric acid as electrolyte
oxidation at anode: Pb + SO42- → PbSO4 + 2e-
reduction at cathode: PbO2 + 4H+ + SO42- + 2e- —> PbSO4 + 2H2O
reverse reaction occurs during charging
advantages
can deliver large amounts of energy fast
disadvantages:
heavy
lead and sulphuric acid are polluting
NiCad cells: secondary
produce 1.2V
nickel as positive electrode, cadmium hydroxide as negative, potassium hydroxide as electrolyte
reaction during discharge:
2NiO(OH) + Cd + 2H2O → 2Ni(OH)2 + Cd(OH)2
process is reversed during charging
advantages:
long life
disadvantages:
cadmium is a toxic heavy metal
low voltage
electrolytic cells
ionic compounds conduct electricity when molten/in solution, the current causes ionic compound to split up and form new substances, this is electrolysis
electrolyte contains positive and negative ions
negative ions move to anode(+) and lose electrons by oxidation
positive ions move to cathode (-) and gain electrons by reduction
e.g electrolysis of molten lead bromide
Pb2+ ions move to cathode (-) where they are reduced
Pb2+ + 2e- —> Pb (s)
Br- ions move to anode (+) where they are oxidised
2Br- —> Br2 + 2e-
redox in cells
electrochemical cells are either voltaic or electrolytic
voltaic cells generate electricity from chemical reactions
spontaneous
electrolytic cells drive chemical reactions using electrical energy
non-spontaneous
reduction always happens at cathode, and oxidation always at anode, but polarity changes for diff types of cells

oxidation of alcohols
primary alcohols can be oxidised to form aldehydes which can be further oxidised to form carboxylic acids, using oxidising agents [O]
if an aldehyde is not distilled off, further oxidation with excess oxidising agent will oxidise it to carboxylic acid
secondary alcohols can be oxidised to form ketones only
requires sustained heating
tertiary alcohols do not undergo oxidation
bc there has to be hydrogen on the functional group carbon which breaks off to make water
only C-C bonds on functional group carbon in tertiary alcohol
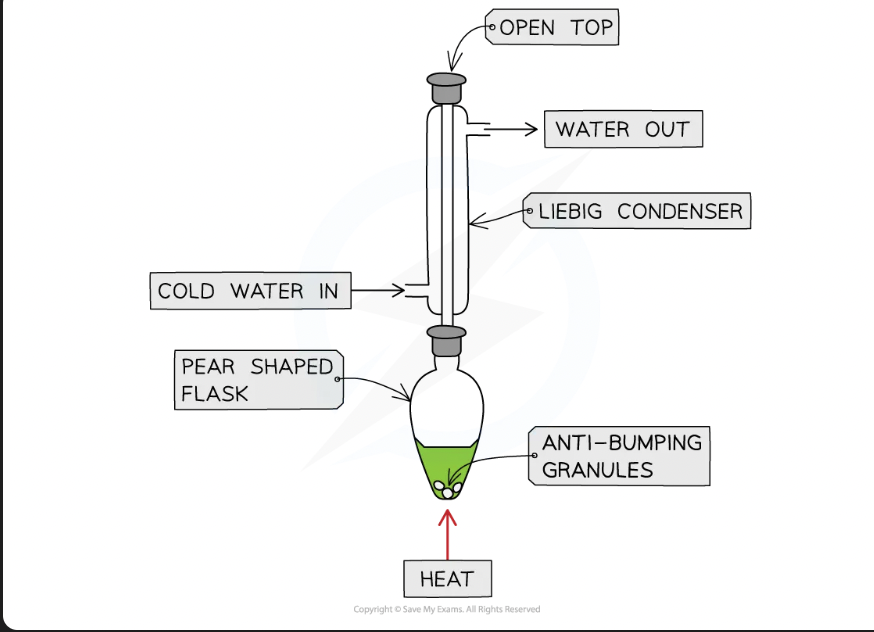
distillation
to make aldehyde from primary alcohol, mixture must be heated
aldehyde product has lower bpt than alcohol (since it lost the H-bonding) so can be distilled off as soon as it forms
distillation carried out using a side arm arrangement which acts as an an air condenser

heating under reflux
for reactions requiring sustained heating
to prevent loss of volatile reactants, apparatus includes condenser positioned vertically, which returns components back into reaction flask
reduction of carboxylic acids, aldehydes and ketones
oxidation reactions an be reversed in presence of a suitable reducing agent [H]
carboxylic acid —> aldehyde —> primary alcohol
ketone —> secondary alcohol
most common reducing agents:
lithium aluminium hydride, in anhydrous acid (stronger, so can reduce carboxylic acids straight to primary alcohols)
sodium borohydride, in aqueous solutions
both of these produce the nucleophilic hydride ion H- which reacts with electron-deficient carbon atom of polar carbonyl group
reduction of unsaturated compounds
reduction of alkene to alkane
addition of hydrogen
requires temp of 200 C, nickel catalyst, and 1000kpa pressure
reduction of alkyne to alkene and alkane
addition of hydrogen
more hydrogen required to make alkane straight from alkyne
degree of unsaturation can be deduced from the structural formula of a molecule
alkenes containing one double bond can be made saturated by adding one mol of H2, so degree of unsaturation is 1
alkynes containing one triple bond can be made saturated by added 2 mol of H2, so degree of unsaturation is 2
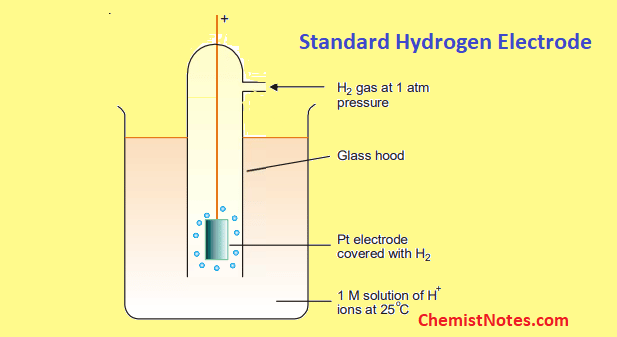
the hydrogen electrode
the absolute value of a half cell potential cannot be measured, only differences in potential between pairs of half cells can
hence, the half-cell used as a reference is the standard hydrogen electrode
under standard conditions and 1.0 mol dm hydrogen ion conc
platinum elecrode used
H + (aq) + e− ⇌ 1/2H2(g)
given the value of Eθ = 0.00 volts
the more negative the Eθ value for a half cell, the better it can act as a reducing agent
standard cell potential
also known as the standard emf, is the difference between the standard Eθ (reducton potential) values of 2 half cells
Eθcell = Eright - Eleft
or Eθcell = Eθred - Eθox
Eθcell = Eθcathode - Eθanode
the half cell with the more negative electrode potential will be anode, more positive is cathode
Eθcell is positive for a spontaneous reaction
gibbs energy and standard cell potential
in electrochemical cells, a spontaneous reaction occurs when the combined half cells produce a positive voltage through the voltmeter, thus:
Eθcell is positive, reaction is spontaneous
Eθcell is negative, forward reaction non-spontaneous but reverse is
therefore: ΔGθ = -nFEθ
n = number of electrons transferred
F = Faraday constant
expressed in kJ mol-1
if both ΔGθ and Eθ are 0, reaction is at equilibrium
electrolysis of aqueous solutions
when aqueous of solutions of ionic compounds are electrolysed, products are complicated to predict as there are additional ions present from the water
at cathode, metal ion M+ or water can be reduced
2H2O + 2e- —> H2(g) + 2OH- (aq)
at anode, anion A- or water can be oxidised
2H2O (l) —> 4H+(aq) + O2 (g)+ 4e-
which species is discharged depends on:
relative values of Eθ
concentration of ions present
identity of electrode
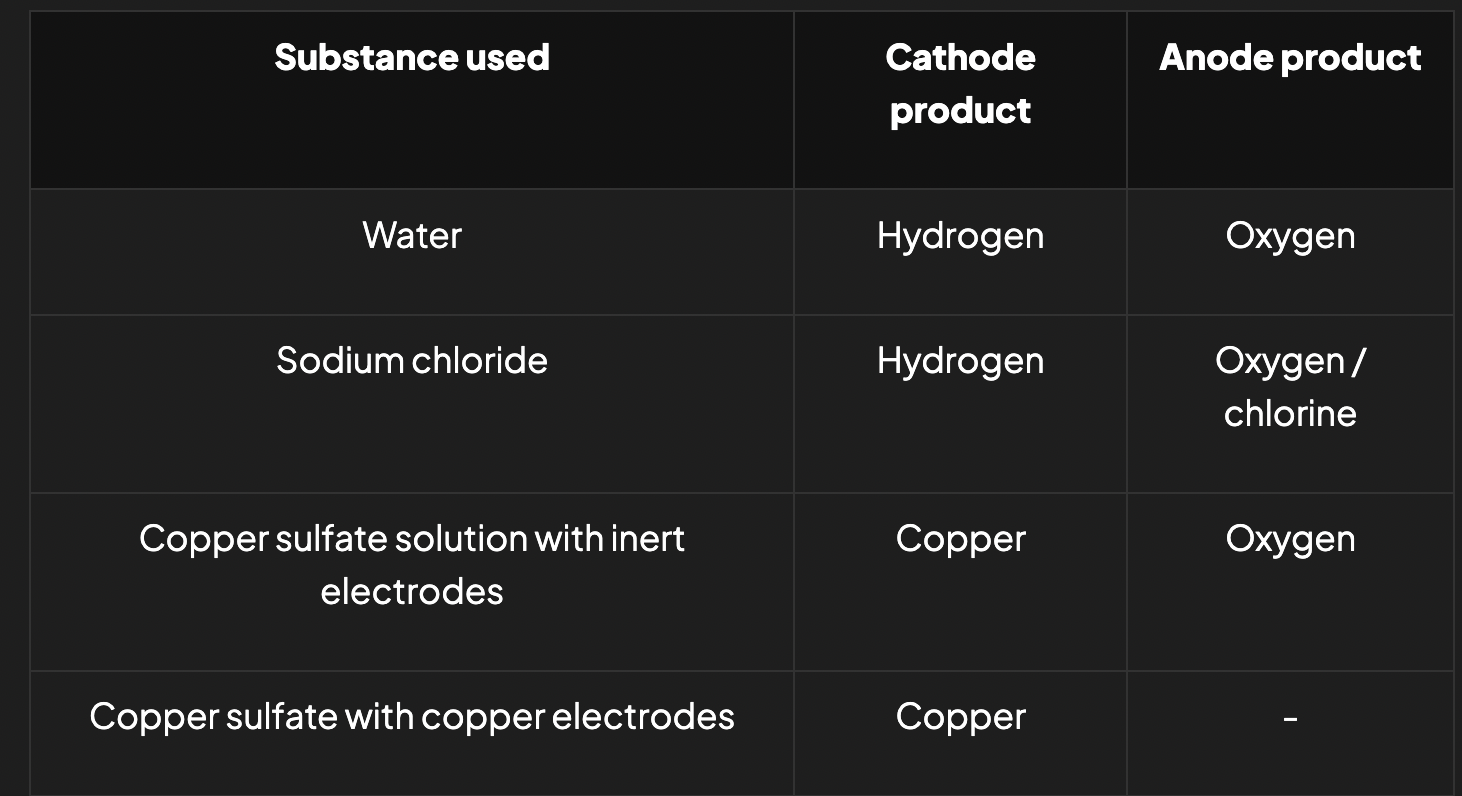
influence of relative Eθ values
the lower the metal ion in the reactivity series (i.e the more positive its standard reduction potential), the more readily it will be reduced to form a metal at the cathode
e.g in the electrolysis of a solution of NaOH, hydrogen will be evolved (reduction of water) at the cathode instead of sodium, because it’s lower in the reactivity
but, in a solution of copper (II) sulfate, copper will be deposited at the cathode in preference to hydrogen
influence of the concentration of ions
if one ion is more concentrated than another, then it is preferentially discharged
e.g when electrolysing aqueous NaCl, both oxygen (from oxidation of water) and chlorine evolved at the anode
for dilute solutions of NaCl, mainly oxygen evolved
for more concentrated solutions of NaCl, more chlorine is evolved
influence of nature of electrode
if copper electrodes are used during electrolysis of copper sulfate, then the anode itself is oxidised to release electrons and form copper (II) ions
since copper is simultaneously deposited at the cathode, the concentration of the solution remains constant throughout electrolysis
electroplating
electroplating involves the electrolytic coating of an object with a very thin metallic layer
anode is usually made from the same metal to replenish the loss of the metal during electrolysis and maintain a constant concentration
the object to be electroplated is attached as the cathode
e.g in copper plating
cathode is the object to be plated
electrolyte is copper (II) sulfate
anode is copper to replenish
as electricity passes through the solution, the copper anode dissolves in the solution forming Cu2+ ions and the Cu2+ in the solution are deposited onto the cathode