EXAM REVIEW: UNIT 1 - BIOCHEMISTRY
1/131
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
132 Terms
Intermolecular Forces
Weak forces that adhere or interact with each other in cell processes, determines the SHAPE of large molecules
Strength of Intermolecular Forces (strongest to weakest)
Ion-dipole
Ion-Induced dipole
Hydrogen bonds
Dipole-Dipole interactions
Dipole-Induced dipole
London dispersion forces
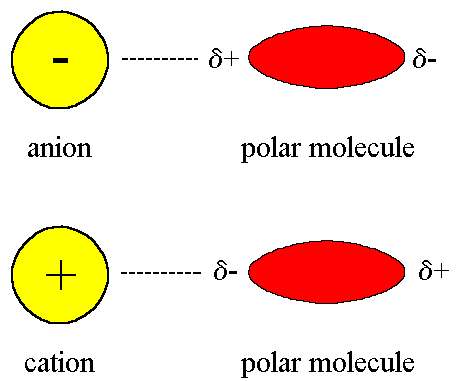
Ion-Dipole
An interaction between an ion (cation or anion) and a polar molecule; the attraction occurs between the ion and the oppositely charged dipole
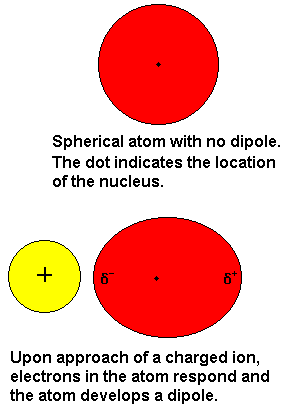
Ion-Induced Dipole
An interaction that occurs when an ion induces a temporary dipole in a nonpolar molecule, leading to an attraction between them.
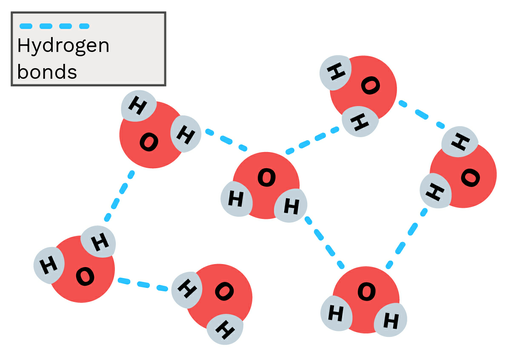
Hydrogen Bonds
Strong attractions between a hydrogen atom, covalently bonded to a highly electronegative atom (HFON), and another electronegative atom.
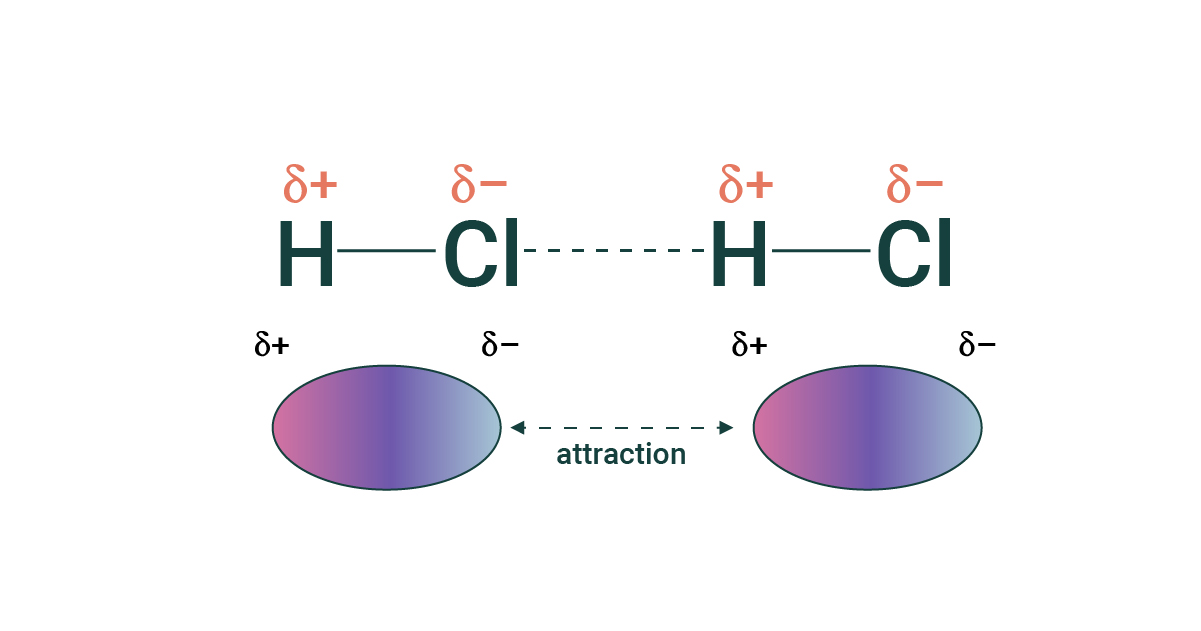
Dipole-Dipole
An interaction between polar molecules where the positive end of one dipole is attracted to the negative end of another dipole.
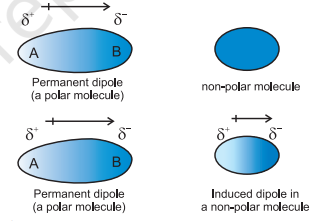
Dipole-Induced Dipole
An interaction that occurs when a polar molecule induces a temporary dipole in a nonpolar molecule, resulting in an attraction between them.
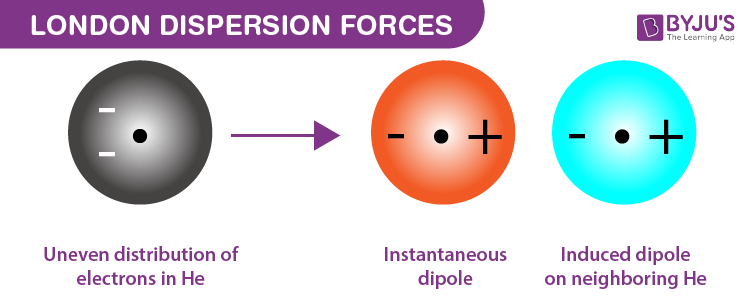
London Dispersion
forces are weak intermolecular forces arising from temporary dipoles in molecules, significant in nonpolar substances.
Properties of Water
Water lattice
High heat capacity
Adhesion
Cohesion
Solid is less dense
Universal solvent
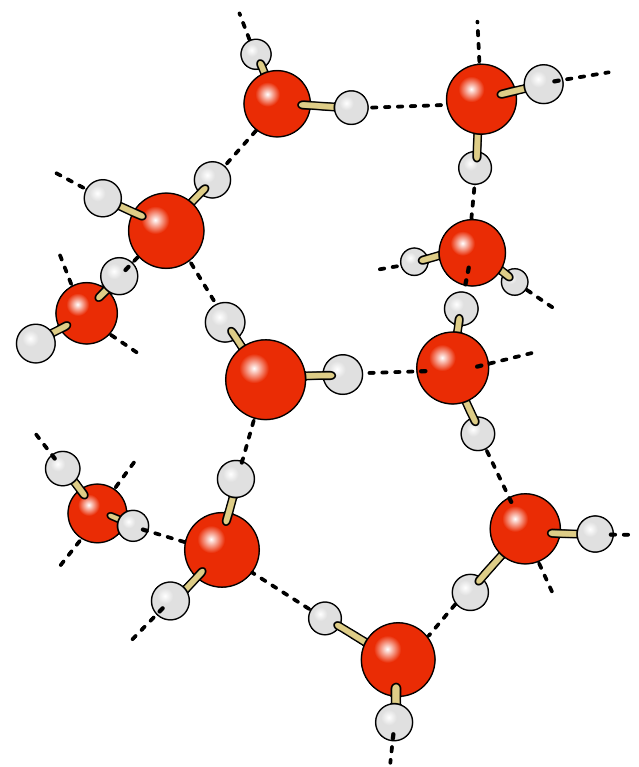
Water lattice
The H-bonds between water molecules allow for a water lattice; bonds break and reform constantly to give water its fluid properties
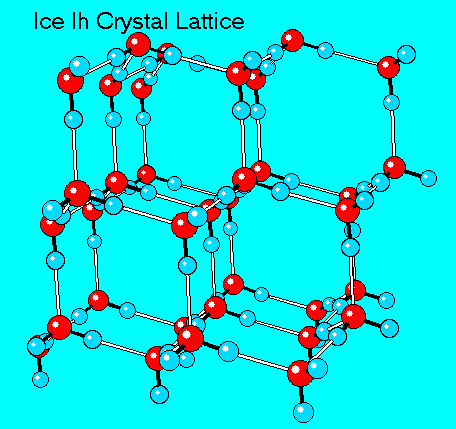
Ice
Water lattice becomes a rigid, crystalline structure with more space between molecules, forming hexagons with air inside of them - it is less dense than water
Water heat capacity
H-bond lattice allows for high heat capacity, thermal energy flows through water and is absorbed by BREAKING H-BONDS which helps regulate temperature.
Universal Solvent
Polar nature of water allows it to dissolve other polar things
Adhesion
The attraction between water molecules and other substances, allowing water to stick to surfaces.
Cohesion
The attraction between water molecules due to hydrogen bonding, which helps maintain surface tension and allows for water's unique properties.
Functional Groups - Definition
Small reactive groups that are usually ionic or polar, causes the chemical reactions of organic molecules, determining their properties and reactivity.
Acid Functional Groups (ionic)
Carboxyl; COOH —> COO-
Phosphate; PO42-
Basic functional Groups (ionic)
Amino; NH2 —> NH3+
All functional groups
Amino
Carboxyl
Carbonyl
Phosphate
Sulfhydryl
Hydroxyl
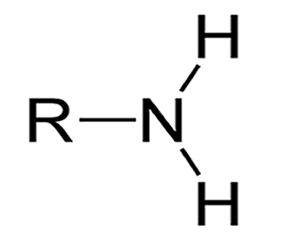
Very EN, forms peptide bonds
Amino
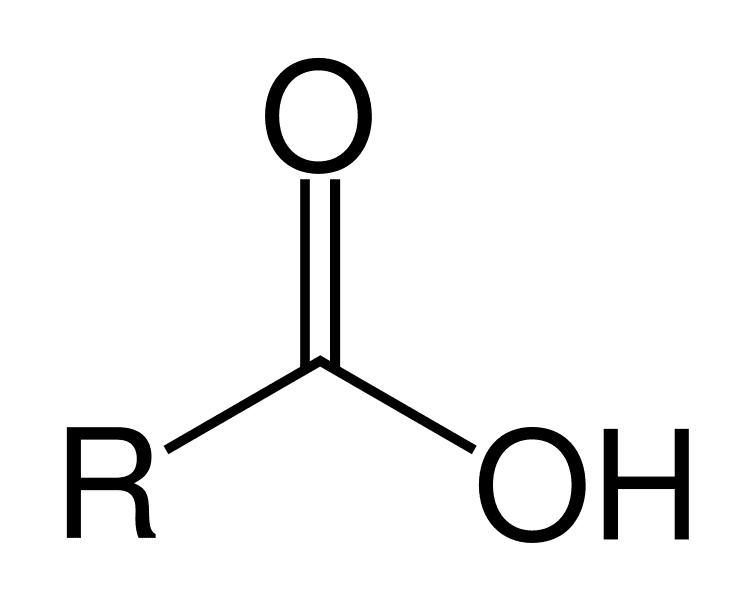
Stronger than phosphate, easy to lose H+, harder to put it back (strong acid; weak conjugate base)
Carboxyl
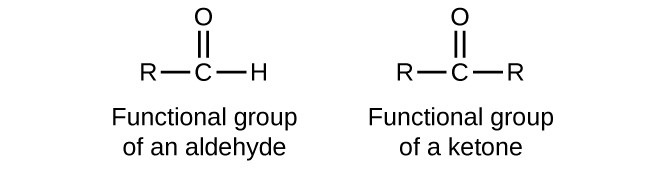
Carbonyl
Aldehyde vs Ketone
Aldehydes are at the end of a molecule; Ketones are within a molecule
Both are CARBONYLS (C = O)
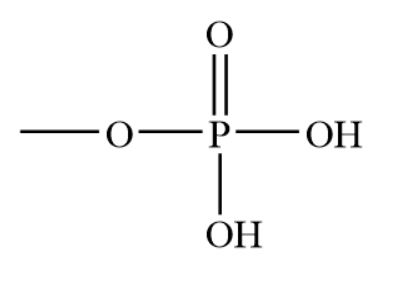
Acts as a buffer, can give/receive H+ so it constantly transitions, stores and releases energy
Phosphate
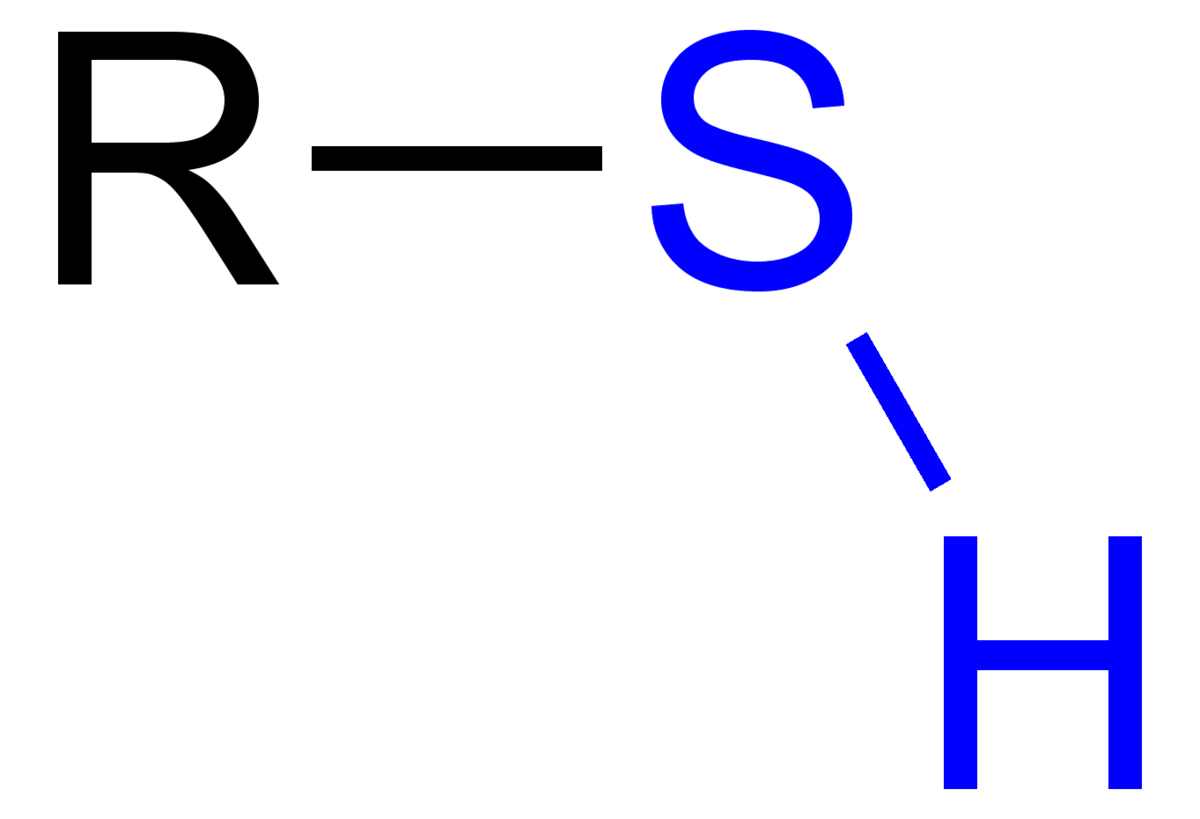
Very reactive; forms disulfide bonds that can withstand acids
Sulfhydryl
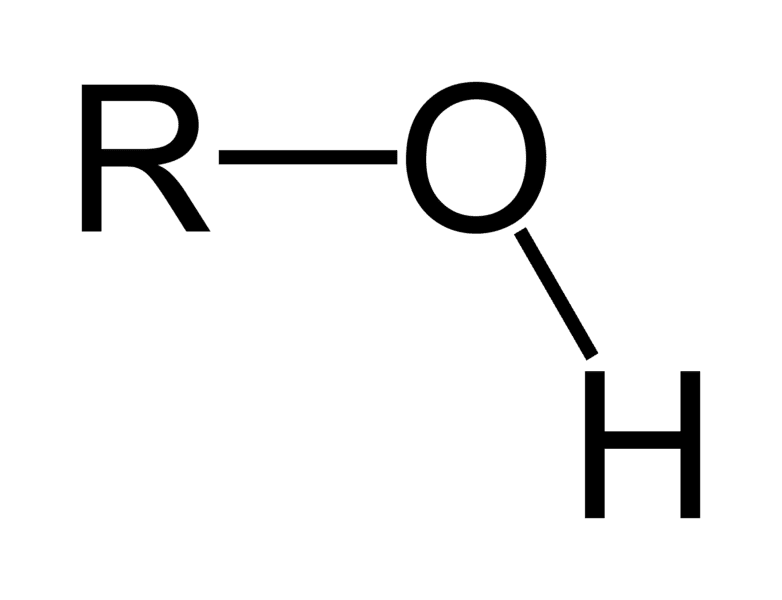
Hydroxyl
What are functional groups essential for
Solubility
Reactivity
Shaping
What can acid/base functional groups do
regulate pH
Ether bonds
C - O - C
Ester bonds
C - O - C = O
Phosphoester (2 means a phosphodiester)
C - O - P = O
Dehydration Synthesis (condensation reaction)
Removes a water molecule to assemble a larger molecule (makes monomers into polymers)
Hydrolysis
Breaks down compounds with the addition of water
Carbohydrates
Short and medium-term energy storage
Short = monosaccharides
Medium = glycogen, starch (polysaccharides)
Build structures
Plant cell walls (cellulose)
Insect shells (chitin)
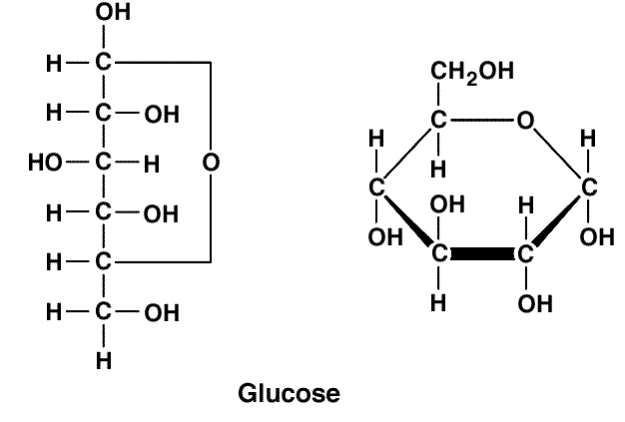
Monosaccharides
3+ carbon sugars, simplest form is glyceraldehyde
Are linear when dry, 5+ carbons will turn into a ring IF IT HAS ALDEHYDE
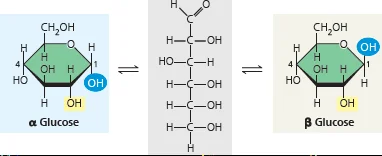
Alpha vs Beta glycosidic bonds
Only occurs if one of the monosaccharide uses C1 in their bond. The alpha and beta is then dependent on the orientation of the hydroxyl on the other monosaccharide’s C1
Alpha - Hydroxyl is DOWN (opposite of chimney)
Beta - Hydroxyl is UP (same direction as chimney)
Carbohydrate functional groups
Hydroxyl
Carbonyl (aldehyde if cyclic, but can also be a ketone)
Carbohydrates - Bonds + linkages
Ether bonds
Glycosidic linkages (between carbohydrates)
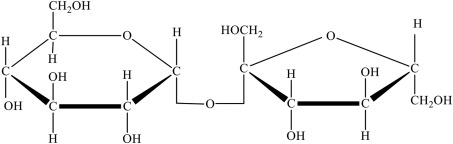
Disaccharides
2 linked monosaccharides connected with a glycosidic bond
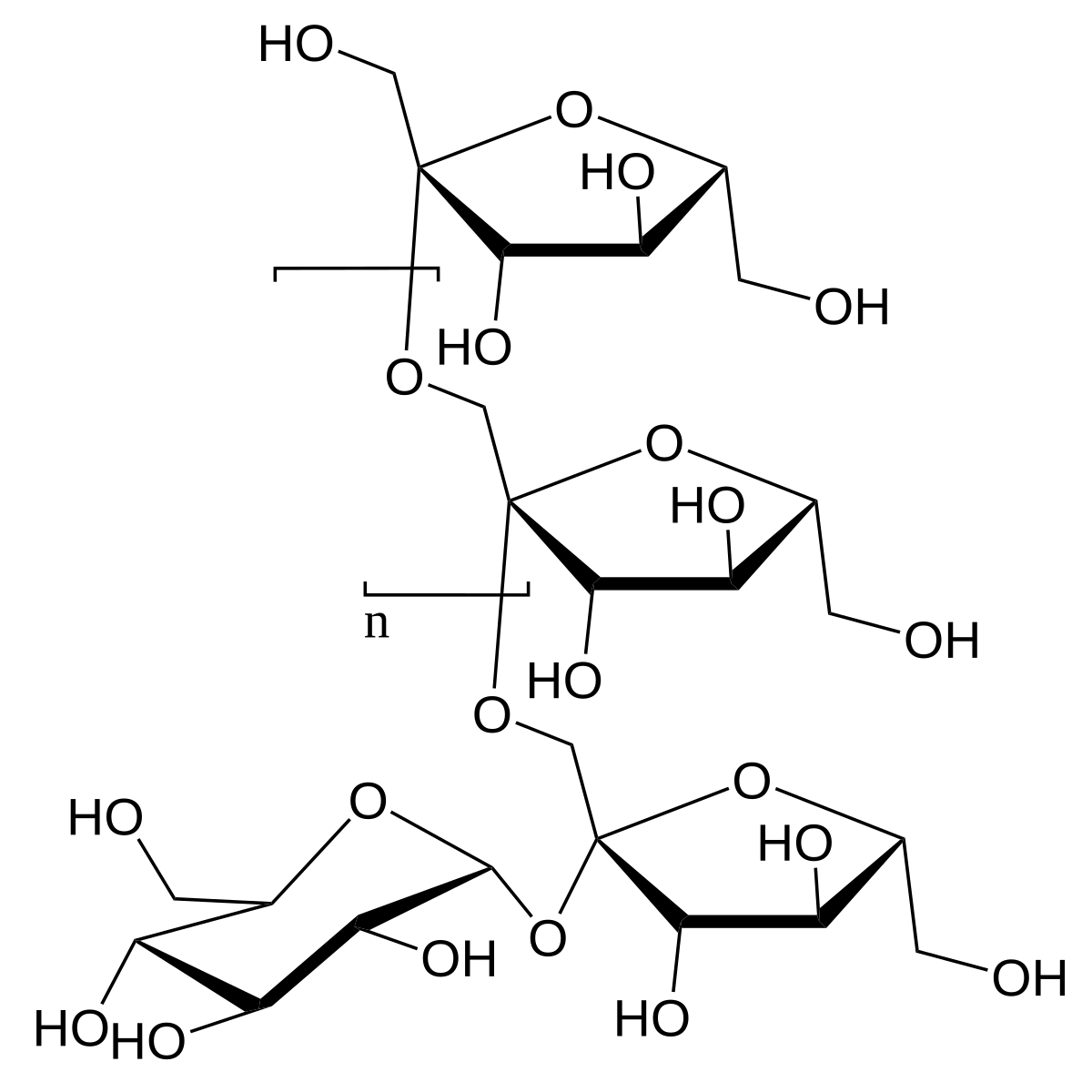
Oligosaccharide
3-10 monosaccharides, acts COMPLEXLY
Has many possible combinations and is VERY VARIABLE
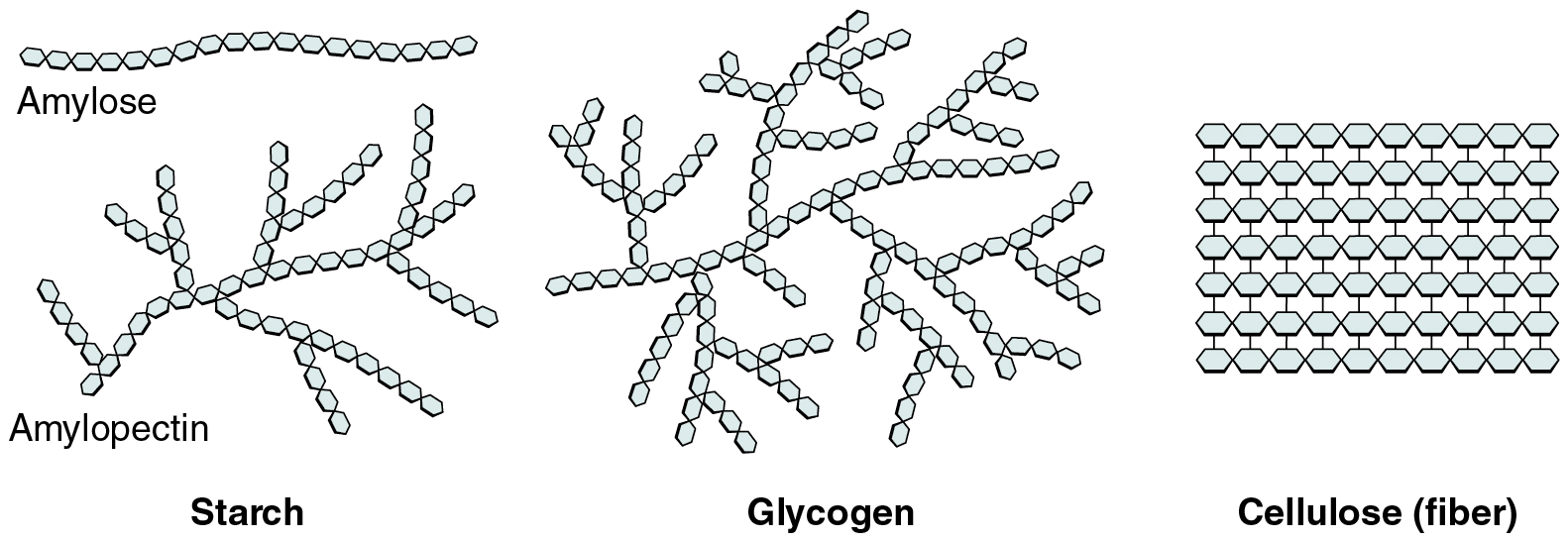
Polysaccharides
Long chains (up to 100s) of monosaccharide repeats - makes complex CARBOHYDRATES, LESS DIVERSE THAN OLIGO (a lot of repetition)
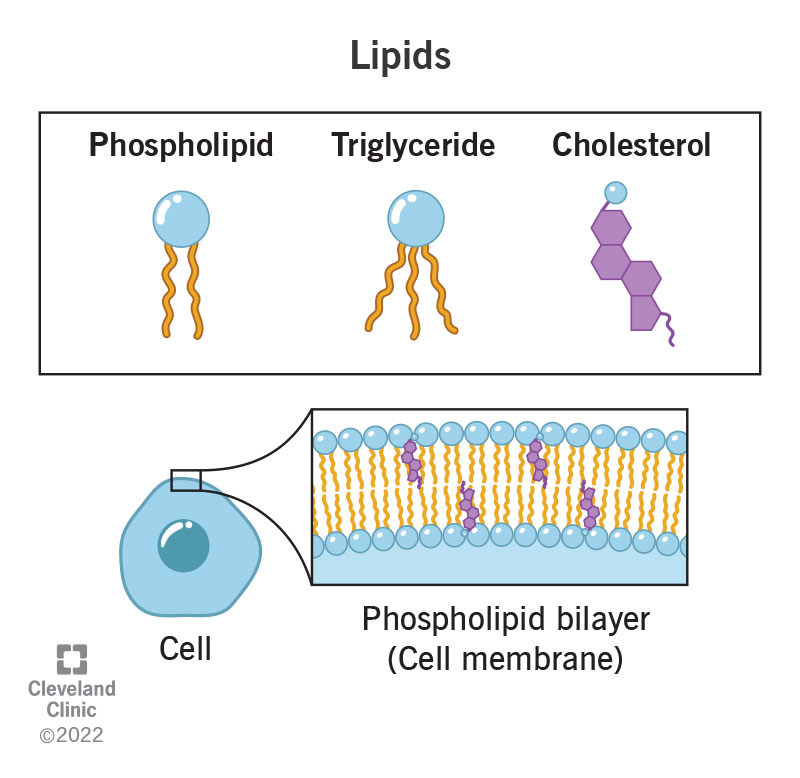
Lipids
Fats, oils, steroids or waxes, typically long chains of non-polar hydrocarbons, (water would rather H-bond with itself)
Ratio of CHO in carbohydrates
1:2:1
Lipids - Function
Cell membranes
Long-term energy storage
Mostly UNREACTIVE
Steroid hormones
Insulation
Organ cushioning
Categories of lipids
Triglycerides
Fatty acids
Phospholipids
Sterols (cholesterol)
Waxes
Lipids - Functional Groups
Carboxyl (fatty acid)
Hydroxyl (on glycerol)
Lipids - Bonds/Linkages
Ester bonds (connects fatty acid to glycerol for triglycerides)
Ether bonds (found within the ether bonds)
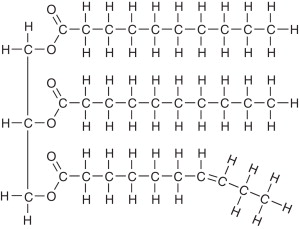
Triglycerides
uses hydroxyls for reactions
Composed of 3 fatty acids and 1 glycerol molecule
Triglycerides - dehydration synthesis
H on hydroxyl is easier to give
OH on carboxyl is easier to give
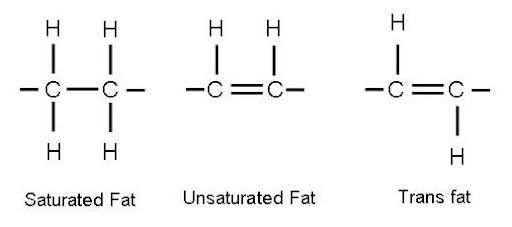
Fatty acids
Can be:
- Saturated (surrounded by H; straight chain)
- Unsaturated (not fully surrounded by H)
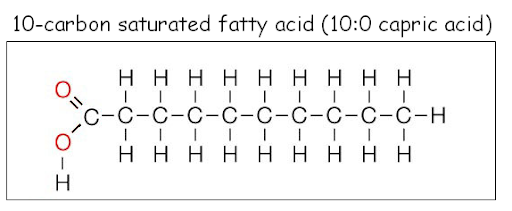
Saturated fatty acids
Tightly packed, straight chain structure that is SOLID AT ROOM TEMPERATURE
Unsaturated fatty acids
Have double bond Cs due to it not being fully surrounded by H, has different types:
cis-unsaturated fats
trans-unsaturated fats
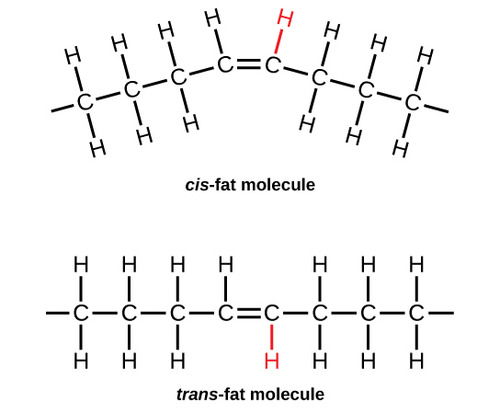
Cis-unsaturated fats
Bent/kinked, liquid at room temp because its shape takes up more space (ex. plant oils)
Bent shape is due to the e- clouds from H ON THE SAME SIDE, repelling each other and bending the molecule
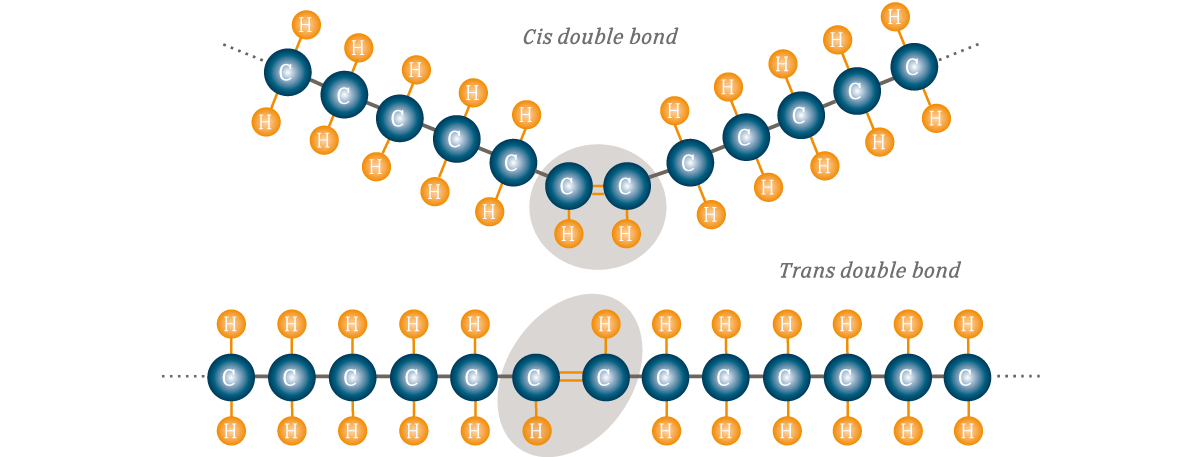
Trans-unsaturated fats
Straight molecules because the e- clouds are on opposite sides, occurs in HYDROGENATION
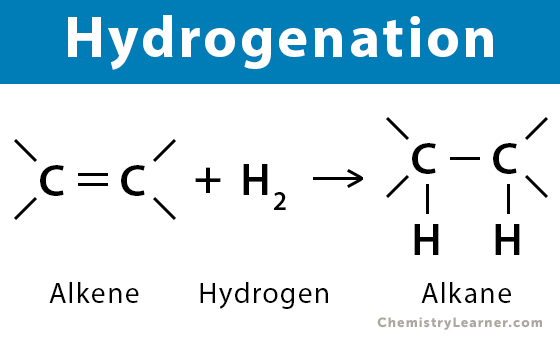
Hydrogenation
Created in the presence of a NICKEL CATALYST; Hs are shot at the molecule until cis —> trans (liquid to solid)
Must be done with POLYUNSATURATED FATS, if not then all Hs would flip, and the fat would be over hydrogenated and unusable
Danger of trans fats
Since mostly artificial, enzymes + bacteria cannot recognize the fat since it cannot be broken down
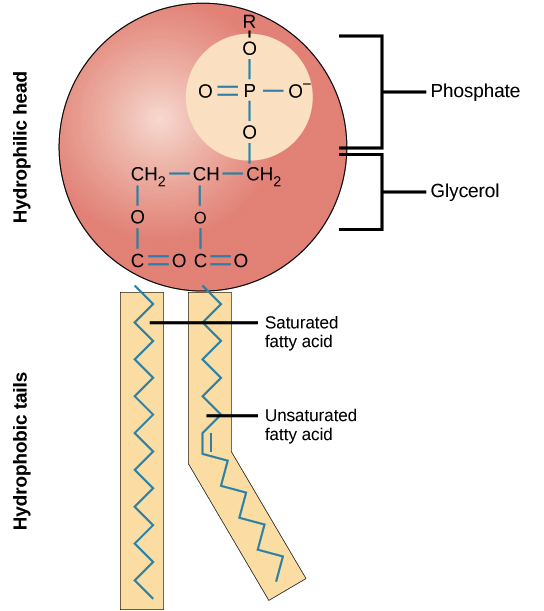
Phospholipids
Similar to triglycerides, but 1 fatty acid is replaced with a phosphate group, the phosphate makes a head that is CHARGED AND POLAR
Phosphoester linkage connects the phosphate
Phospholipid - dehydration synthesis
Phosphate loses OH
Glycerol loses H
Phospholipid - fatty acid tails
Fatty acid tails are nonpolar, they stay together but are on the other side of the phosphate
Type of fatty acid depends on temperature, if stiff is needed: 2 saturated fatty acids, if fluid is needed: 1 saturated 1 cis monounsaturated
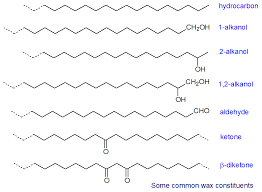
Waxes
Long hydrocarbon chains (2-3 fatty acids linked together) formed by an ester link between a long chain alcohol and fatty acid - COMPLETELY NONPOLAR
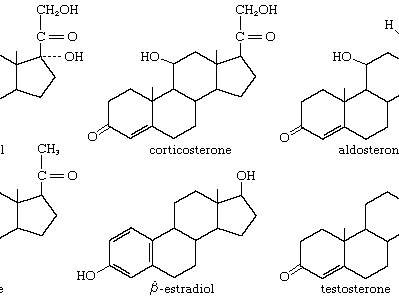
Steroids (cholesterol)
Only lipids with a multi-ring structure, CHOLESTEROL is a precursor to all steroids
Needed for membrane integrity, to get the ideal fluidity (wedges between or sticks phospholipids together to adjust based on temperature)
Proteins
Long polymers of ~50 amino acids, consists of CHON, and is NEUTRAL, the function is determined by the R-group
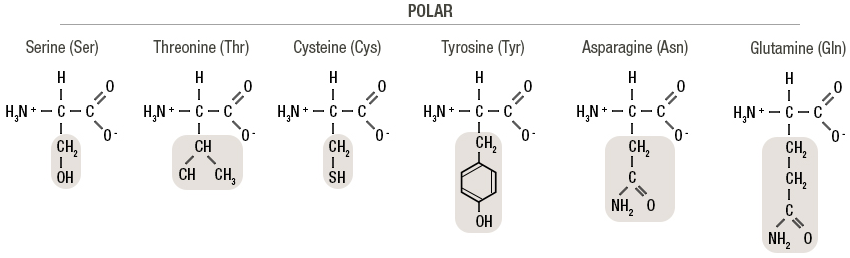
Polar proteins (nucleophilic)
Hydroxyl (OH) or suldhydryl (SH)
Nonpolar proteins
Neutral charge across the molecule, may have an S in between the lines
Acidic proteins
Has a second carboxyl APART FROM THE ONE FROM THE PEPTIDE BOND
Basic proteins
Has N outside of the N in the amines
Aromatic proteins
Has a hexagon within its structure
What determines the function of proteins
SHAPE - which is determined by the R-groups, the ones sticking out have the potential to react
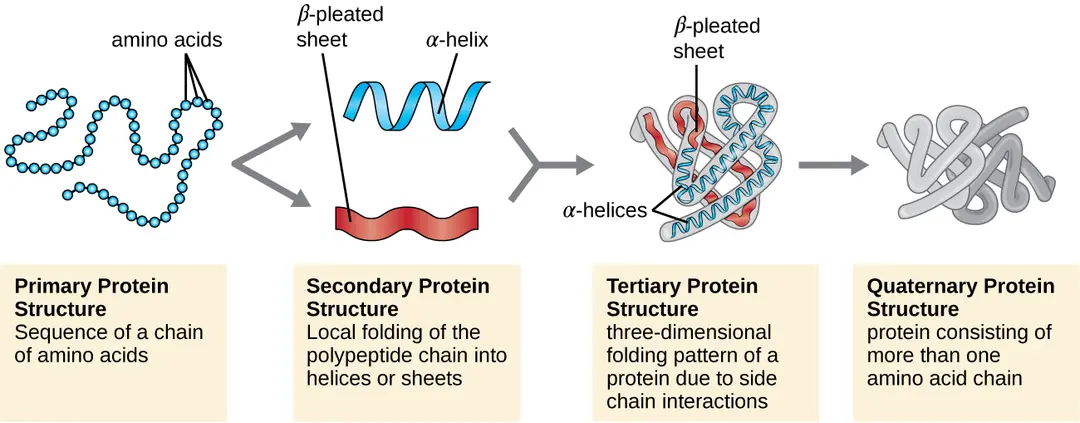
Stages of protein folding
Primary (peptide chain)
Secondary (a helix or B sheet)
Tertiary (functional protein)
Quaternary (complex unit)
Primary folding
Forms a polypeptide chain by linking amino acids together with covalent peptide bonds - dehydration synthesis between AMINE AND CARBOXYL —> amino acids with peptide bonds
Secondary folding
Depending on the properties of R-groups, peptide bonds may interact with H-bonds to make:
alpha helix - inside is nonpolar, some R-groups are nonpolar and are within the helix
beta pleated sheet - R groups surround the hydroxyl
Polar and nonpolar R-groups are neither shape, just a chain
Tertiary folding
Final 3D shape formed by R-groups interacting with intermolecular forces + covalent bonds, more disulfide bonds = more heat and acid resistant
Quaternary folding
Multiprotein complex that are formed with the remaining R-groups, only done if the shape and R-groups allow it
Protein - functions
Structural components of tissues
Enzymes
Protein hormones
Channels for transport
Antibodies in immune system
Nucleic Acids
Formed from phosphates, CHONP, make different coded instructions for the cell, DNA is long polymers of 4 different types of nucleotide subunits
Components of nucleotides
1 sugar molecule (ribose or deoxyribose)
1 phosphate group
Nitrogenous base
Bonds/linkages in nucleotides
Phosphodiester
Ester
Ether
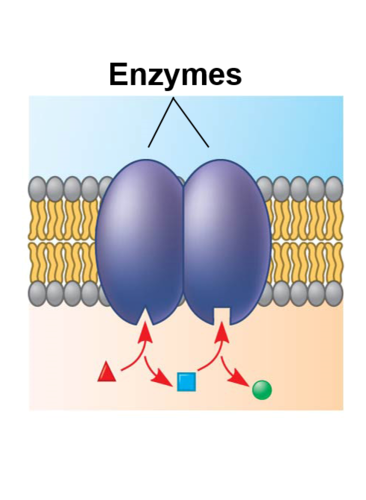
Enzymes
Catalysts that speed up chemical reactions by reducing activation energy
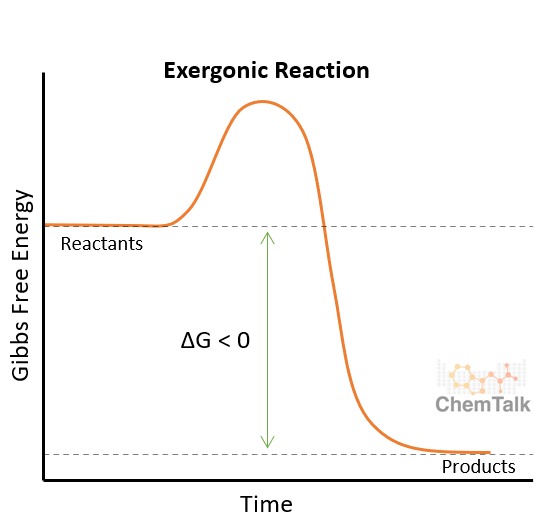
Spontaneous reactions - EXERGONIC
Decrease in free energy; cellular respiration
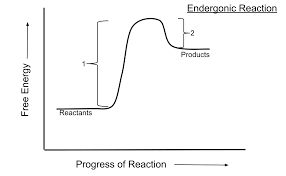
Nonspontaneous reactions - ENDERGONIC
Increase in free energy; photosynthesis
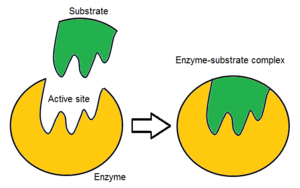
Active site
Where reactions occur in an enzyme - substrates bind here
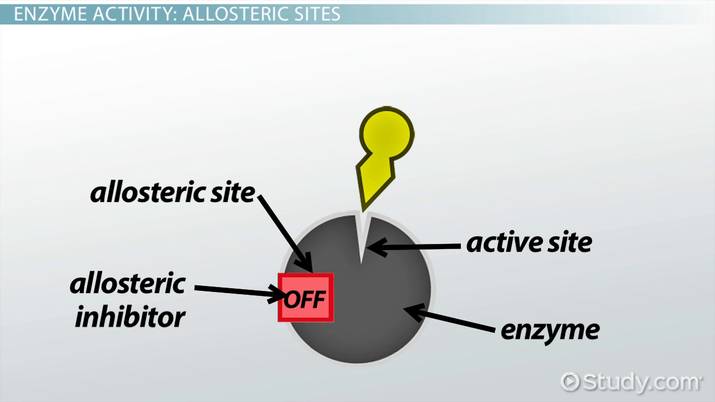
Allosteric site
Another site on an enzyme where activation or inhibition may occur
Allosteric Activation
A molecule interacts with the allosteric site, changing the enzyme’s shape and allowing it to fit the shape of the substrate
Cofactor/Coenzyme activation
Organic molecules (coenzymes) or ions (cofactors) that complete the active site
Competitive inhibition
Competitive inhibitors interact, but DO NOT REACT with the enzyme, the rate of reaction decreases
Allosteric inhibition
Enzymes work or don’t work; the interaction with the allosteric site changes the shape of the enzyme, cell chooses when to make something to pull the inhibitor out
Competitive allosteric
Changes the shape of the enzyme TOO MUCH, active site is smaller
Noncompetitive allosteric
Inhibitor interacts with the enzyme and changes its shape, but not enough so the substrate can have a reaction - molecules will INTERACT but will not REACT
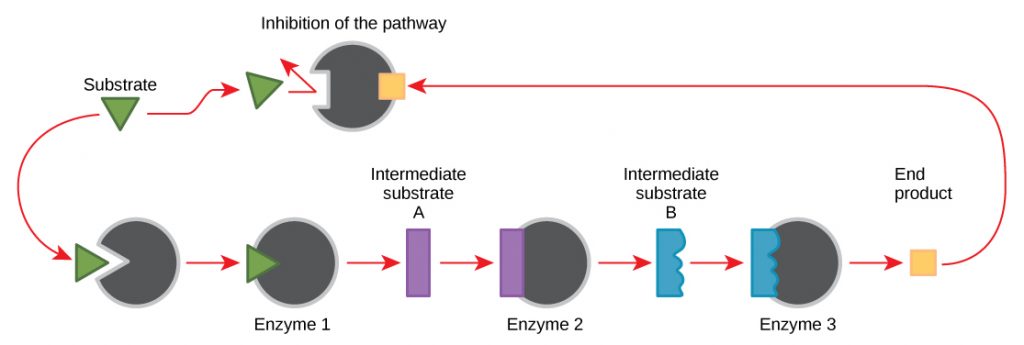
Feedback inhibition
A regulatory mechanism where the end product of a pathway inhibits an enzyme that was earlier on in the chain; prevents overproduction of substances and helps maintain homeostasis
Catabolic reactions
Break molecules down into smaller and simpler ones, releasing energy in the process
Anabolic reactions
Construct larger molecules from smaller, simpler units; require energy input
Endothermic reactions
Absorb heat from surroundings, decrease in temperature; energy of products is higher than reactants (endergonic)
Exothermic reactions
Release heat into surroundings, increase of temperature in the environment; energy of products is lower than reactants (exergonic)
Plasma membrane - functions
Boundary that separates inside of cell from its surroundings
Selective permeability allows certain substances to pass through and not others
Fluid mosaic model
Membrane is continuously shifting/drifting with a variety of molecules attached to or embedded in it; almost everything moves
Structure of Cell membrane
Phospholipids
Cholesterol
Proteins
Oligosaccharides (identifiers)
Protein function in cell membranes
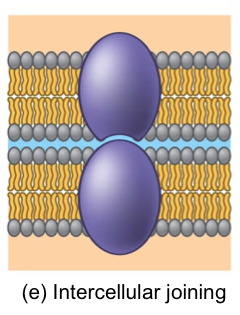
Intercellular joining
Enzymatic activity
Transport (active/passive)
Cell-cell recognition
Anchorage/attachment
Signal transduction
Intercellular joinings
Cells are joined together, they still are separate entities, but can collectively create things like tissues
Enzymatic activity
Molecules enter, their shape gets changed, and newly shaped molecules leave
Passive Transport
Things pass through freely - they move down a concentration gradient, types include:
Osmosis
Diffusion
Facilitated diffusion