Gram Positive Bacteria
1/221
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
222 Terms
how is staphylococcus classified morphologically?
gram (+) cocci occurring in pairs, short chains, and grape-like clusters
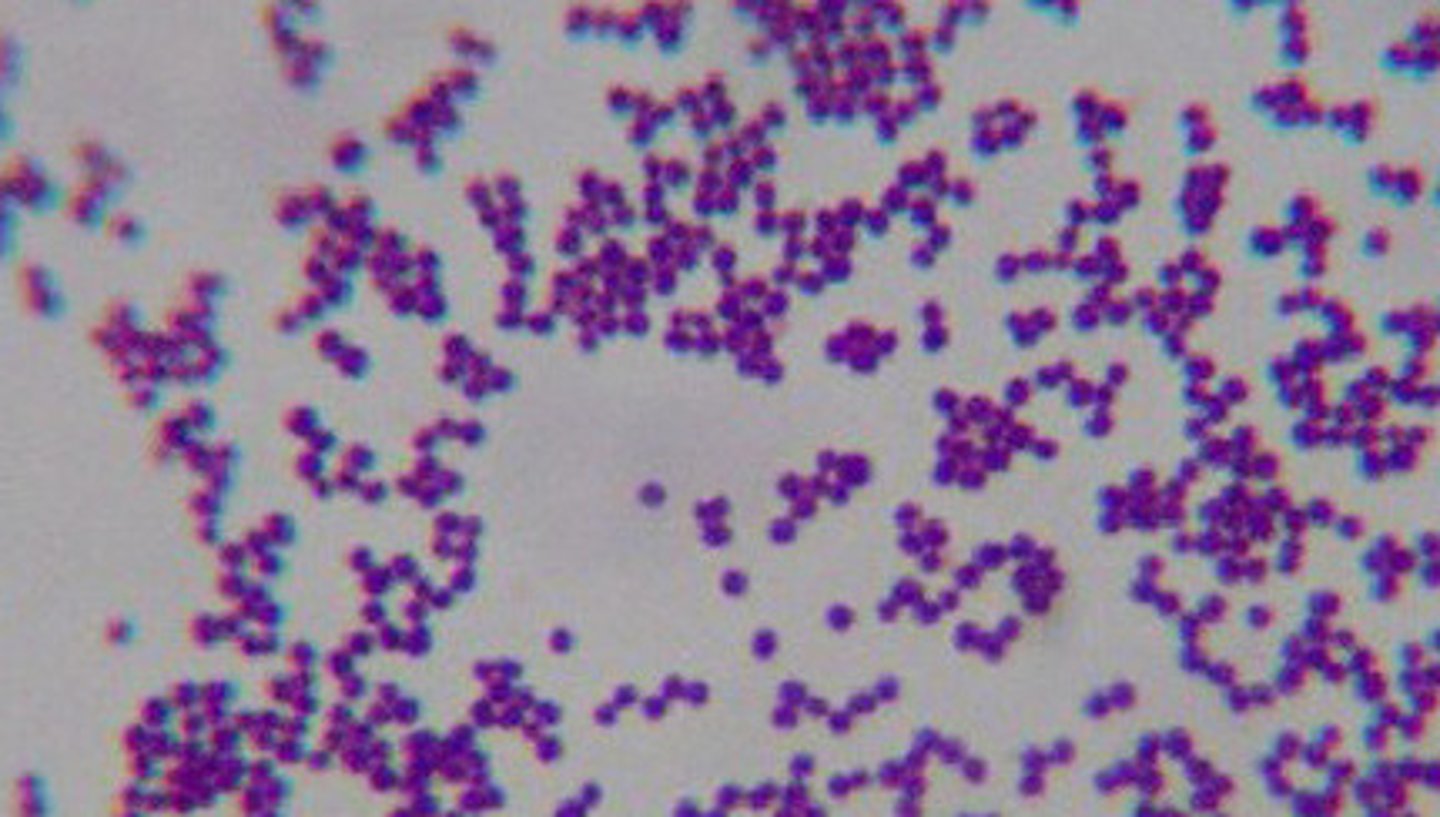
what are the 3 strains of staphylococcus that are most relevant to veterinary medicine?
s. aureus, s. hyicus, s. intermedius
T or F: staphylococcus is highly susceptible to dry environments
F (staphylococcus is highly resistant to dry environments)
what does staphylococcus produce to cause disease in the host?
toxins
what are some examples of diseases that staphylococcus can cause?
mastitis, bumblefoot, greasy pig disease, pyoderma
what is the public health significance of s. aureus?
there is a superbug that is resistant to methicillin (MRSA)
what types of infections does staphylococcus cause?
suppurative (pus-forming)
are staphylococcus aerobic or anaerobic?
facultatively anaerobic
does staphylococcus form spores?
no
are staphylococcus motile?
no
what is alpha hemolysis?
partial hemolysis
what is beta hemolysis?
complete hemolysis
what is gamma hemolysis?
no hemolysis
what causes alpha hemolysis?
beta hemolysin
what causes beta hemolysis?
alpha hemolysin
what result does s. aureus have on the catalase test?
positive, which means it can degrade hydrogen peroxide into water and oxygen, making bubbles in 3% hydrogen peroxide solution
what is the pathological significance of s. aureus having a positive catalase test?
it can deactivate the peroxide radicals, which allows it to survive unharmed in the host
what is the result of a mannitol salt agar with s. aureus?
produces yellow colonies with yellow zones
if an organism can ferment mannitol, an acidic byproduct is formed that will turn the phenol red agar to yellow
why is a mannitol salt agar selective for staphylococci?
the level of NaCl in this test is inhibitory to most other bacteria
what is the pH range for phenol red?
6.4-8.0
what is the pH of neutral red?
4
T or F: staphylococcus is part of the normal flora on skin of most animal species
A (common in nares of humans and some animals)
who is most susceptible to staphylococcus disease?
infants and debilitated adults
what types of lesions does staphylococcus cause? where?
abscesses in almost any body location (even bone marrow)
favored by poor hygiene
what 2 enzymes does staphylococcus use to help it survive in the host?
coagulase and kinase
coagulase: clots blood plasma to form a protective barrier around the bacteria
kinase: dissolves the clot and releases the bacteria
what is a coagulase test?
Tests for the conversion of fibrinogen to fibrin, which walls bacteria from immune response
staphylokinase
potent thrombolytic agent and degrades fibrinogen
leucocidin kills?
WBCs
what types of toxins does staphylococcus produce?
exfoliative toxins (scalded skin syndrome and greasy pig disease)
what "spreading factor" does staphylococcus produce? what does it do?
hyaluronidase catalyzes the degradation of hyaluronic acid so that the bacteria can invade deeper tissues
Explain the superantigen produced by toxic shock syndrome toxin
it is the non-specific activation of T cells resulting in polyclonal T cell activation and massive cytokine release
what are some risk factors that contribute to a host's susceptibility to staphylococcus?
injury to normal skin, viral infections, use of broad-spectrum antibiotics
which strain of staphylococcus affects ruminants? what disease does it cause?
s. aureus causes mastitis in ruminants
which strain of staphylococcus affects poultry? what disease does it cause?
s. aureus causes bumblefoot in poultry
which strain of staphylococcus affects pigs? what disease does it cause?
s. hyicus causes greasy pig disease
which strain of staphylococcus affects dogs? what disease does it cause?
s. aureus causes pyoderma, papules, pustules, and furuncles in dogs
which strain of staphylococcus affects rabbits? what disease does it cause?
s. intermedius causes abscesses and septicemia in rabbits
what is the drug of choice for staphylococcus infection?
penicillin g
antibiotic resistance testing is very important!
what strands of staph are zoonotic?
S. aureus and S. hyicus
how is streptococcus classified morphologically?
fastidious, pyogenic cocci
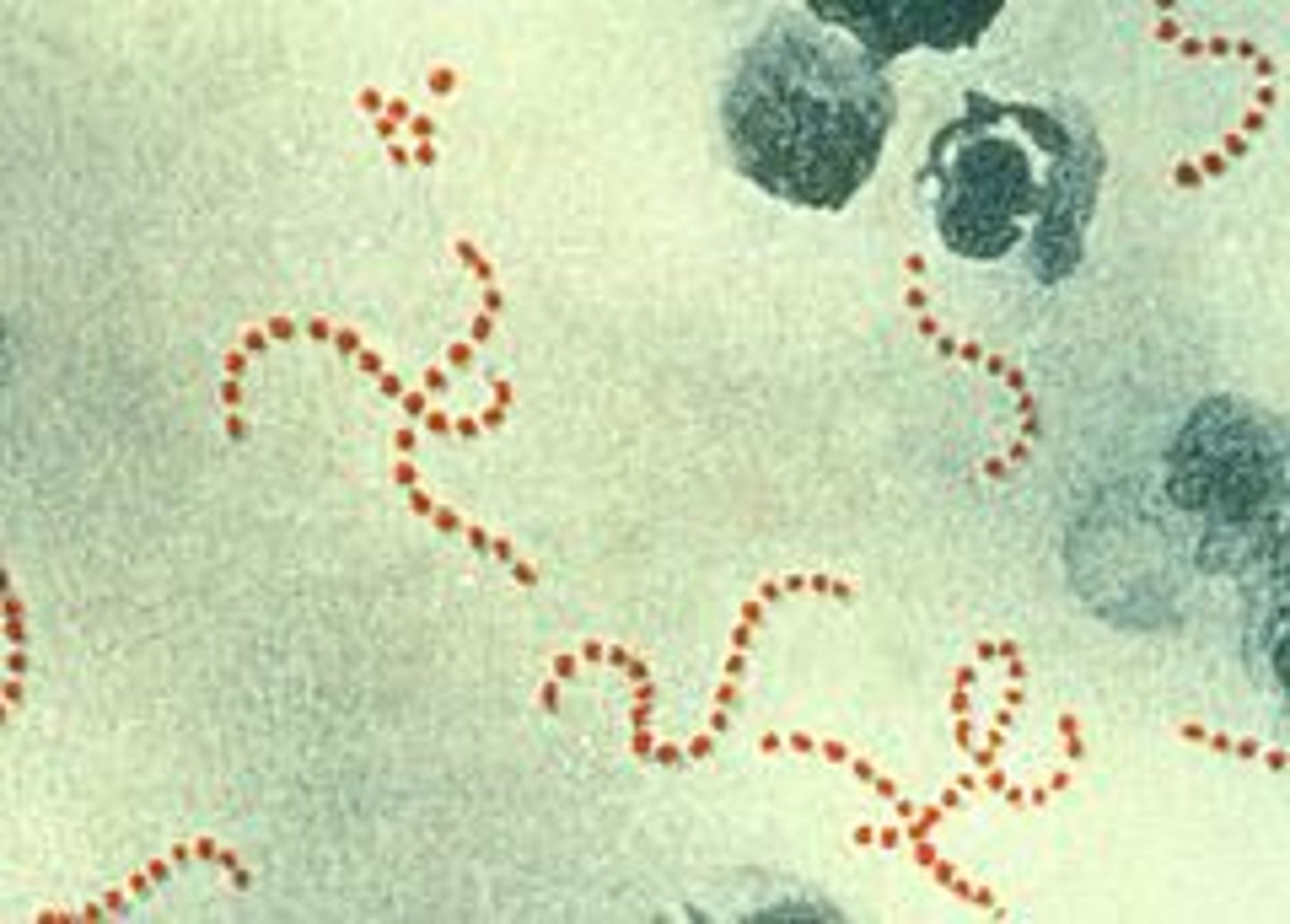
what is the result of a CAMP test with streptococcus?
positive - s. agalactiae acts synergistically with s. aureus to cause enhanced RBC lysis
T or F: streptococcus is typically present in the tonsils and throat, but does not persist in the environment
A
how do we type streptococcus?
Lancefield grouping and M protein
what are the strains of streptococcus that are most relevant to veterinary medicine?
s. pyogenes, s. agalactiae, s. equi, s. suis, s. tonsils, s. canis
what types of infections does streptococcus cause in the host?
localized and systemic infections - throat pain, mastitis, strangles, jowl abscess, pyogenic infections
which strains of streptococcus produce beta hemolysis?
s. pyogenes and s. agalactiae
which strains of streptococcus produce alpha hemolysis?
s. pneumoniae
which strains of streptococcus produce gamma hemolysis?
s. dysgalactiae
what is Lancefield grouping?
a way of typing streptococcus based on surface carbohydrate antigens
24 groups: A-H, K-V
which strain (and serogroup) of streptococcus affects humans? what disease does it cause?
s. pyogenes (A) causes throat pain and fever in humans
which strain (and serogroup) of streptococcus affects cows? what disease does it cause?
s. agalactiae (B) causes mastitis in cows
which strain (and serogroup) of streptococcus affects horses? what disease does it cause?
s. equi (C) causes strangles in horses
which strains (and serogroups) of streptococcus affects pigs? what diseases do they cause?
s. suis (D) causes systemic infections in pigs
s. porcinus (E) causes jowl abscesses in pigs
which strain (and serogroup) of streptococcus affects dogs and cats? what disease does it cause?
s. canis (G) causes pyogenic infection of the anal mucosa in dogs and cats
how is streptococcus serogroup A further subdivided?
serogroup A is further separated into 60 groups based on M (membrane) protein, a type-specific protein antigen
what does M protein do for streptococcus?
inhibits phagocyte binding, toxic to phagocytic cells, facilitates adherence to host cells
what 2 toxins does streptococcus produce? what do these do?
streptolysins S and O
S - oxygen-stable, non-antigenic hemolysin that disrupts lysosomes
O - oxygen-labile, antigenic hemolysin that binds to cholesterol
what "spreading factors" does streptococcus use?
hyaluronidase, DNAase, streptokinase (digests clots)
how is streptococcus transmitted?
droplets or direct contact
which strains of streptococcus cause localized disease but not systemic disease?
s. agalactiae and s. porcinus
which strains of streptococcus cause both localized and systemic disease?
s. pyogenes, s. equi, s. suis, and s. canis
basically, all of them except s. agalactiae and s. porcinus
what is the most common cause of mastitis in lactating cattle? which cells do they colonize? how does it spread among hosts?
s. agalactiae (B) colonizes the epithelial cells in ruminant mammary glands and is spread by milker's hands or contaminated milking machines
how does s. equi affect horses? how does It spread among hosts?
s. equi (C) causes strangles, genital, and mastitis in horses, and is spread by nasal discharge, pus from an abscess, or by contaminated food/water
how does s. suis (D) affect pigs?
fatal pneumonia, fatal acute meningitis, endocarditis, myocarditis, polyarthritis, genital infections and abortions
how does s. canis (G) affect dogs and cats?
pyogenic infections (genital, skin, and wound), metritis, vaginitis, neonatal bacteremia, lymphadenitis
mycoplasma is one of the (largest/smallest) free-living bacteria
smallest
it is also very pleomorphic and filterable, with a fried-egg appearance
T or F: mycoplasma is not susceptible to desiccation and detergents
B (mycoplasma is susceptible to these)
what parts of the host do mycoplasma infect?
respiratory, urogenital epithelium, mastitis, arthritis, conjunctivitis
what is the public health significance of mycoplasma?
contagious bovine pleuro pneomoniae spreading in the US led to the USDA's establishment in 1884
do mycoplasma have a cell wall?
no, this is what makes them pleomorphic, spherical to filamentous cells
this is also why treating mycoplasma with cell wall synthesis inhibitors is a waste of time (instead, use protein synthesis inhibitors and DNA synthesis inhibitors)
T or F: mycoplasma grow very slowly, with a generation time of up to 9 hours
A
what does mycoplasma's cell membrane contain?
sterols
mycoplasma's primary colonization site is -
epithelium
what causes mycoplasma infections to go from clinically silent to clinically infected?
environmental factors, stress, and synergistic infections
how is mycoplasma transmitted?
aerosol
which strain of mycoplasma causes contagious bovine pleuropneumonia?
m. mycoides ss. mycoides
which strains of mycoplasma cause pneumonia, arthritis, mastitis, and abortion in cattle?
m. bovis and m. californicum
which strain of mycoplasma causes vaginitis?
ureaplasma diversum
how are bacteria causing bovine mycoplasmal mastitis shed?
milk, urine, feces, and respiratory secretions
transmitted by milking procedures
what are the clinical signs of bovine mycoplasmal mastitis?
sudden drop of milk, rapid involvement of multiple quarters, swelling, milk is viscous and yellow, fibrosis may cause permanent loss of milk production
which strains of mycoplasma cause enzootic pneumonia and arthritis in pigs?
m. hyopneumoniae, m. hyorhinis, m. hyosynoviae
which strains of mycoplasma cause pneumonia, arthritis, and mastitis in sheep and goats?
m. mycoides, m. mycoides ss. capri, m. agalactiae
which strains of mycoplasma cause chronic respiratory disease in poultry?
m. gallisepticum, m. synovial, and m. meleagridis (turkeys)
what are the clinical signs of chronic respiratory disease caused by mycoplasma in poultry?
cough, nasal discharge, and air sacculitis
usually only fatal if co-infected with other agents
how can you treat mycoplasma?
macrolides, tetracyclines, spectinomycin, and spiramycin
highly sensitive to disinfection!
chlamydia is an obligate - bacteria
intracellular
what is unique about chlamydia's developmental cycle?
elementary bodies and reticulate bodies
which strains of chlamydia are most relevant to veterinary medicine?
c. psittaci, c. trachomatis, c. pneumoniae, c. pecorum, c. abortus
since antibodies are useless against chlamydia, what type of immunity is required?
Th1 immunity
T or F: chlamydial disease is the result of the first infection
B (disease is the result of repeated infection)
how are elementary bodies different from reticulate bodies? (chlamydia's developmental cycle)
EBs are small, metabolically inactive, do not multiply, and are infectious
RBs are large, metabolically active, multiply intracellularly, and are non-infectious
where does chlamydia infect the host?
mucosal epithelia and macrophages
how is chlamydia transmitted?
contact, inhalation, or ingestion
c. psittaci and c. pecorum - sexual transmission
what are the clinical signs of the chronic form of a chlamydial infection?
mononuclear interstitial granulomatous lesion composed of macrophages, lymphocytes, and a few polymorphonuclear leukocytes
what are the clinical signs of the acute form of a chlamydial infection?
polymorphonuclear leukocytes are more pronounced than in the chronic form
how do chlamydiae travel around inside the host?
they infect macrophages and travel with them to multiple sites
which strain of chlamydia causes avian psittacosis and ornithosis? how is it transmitted?
c. psittaci
inhalation and ingestion of elementary bodies
how does chlamydia psittaci affect its hosts?
systemic infection, replication in the respiratory tract (bacteremia), pericarditis, perihepatitis, airsacculitis
how does chlamydia psittaci affect turkeys?
depression, loose sulfur-colored diarrhea
how does chlamydia psittaci affect parrots?
yellow-green diarrhea, cerebral hemorrhage, airsacculitis