Chapter 18: Chemical Bonding Study Guide
1/40
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
41 Terms
The forces that hold different atoms or ions together are
b. chemical bonds.
Each molecule of hydrochloric acid, HCI, contains one atom of hydrogen and
a. one atom of chlorine.
Which compound is formed from a tight network of oppositely charged ions?
d. salt, NaCl
Often atoms join so that each atom will have
b. an outermost energy level that is full of electrons.
When two hydrogen atoms bond, the positive nucleus of one atom attracts the
c. negative electron of the other atom.
An ionic bond is a bond that forms between
a. ions with opposite charges.
Covalent bonds are formed between
c. nonmetal atoms.
Solid ionic compounds have very high melting points because they
d. contain charged ions that are locked tightly together,
The anion formed from an oxygen atom is called a(n)
b. oxide ion.
The name dinitrogen tetroxide tells you that this compound contains
c. Two nitrogen atoms and four oxygen atoms
Fe²O³ (Subscripts) is named iron (III) oxide because it contains
B. Fe³ ions
When copper combines with oxygen to form copper (II) oxide, the charge of the copper ion is
b. Cu²+
The name for the compound with the formula CuBr² (subscript) would be written as
a. copper(II) bromide
The name for the compound with the formula Cr²O³ (subscripts) would be written as
d. chromium(lll) oxide.
It is possible for different covalent compounds to have the same empirical formula because empirical formulas represent
d. a ratio of atoms in the compound
A carbon atom can bond to four other atoms because it has
b. four valence electrons
The simplest organic compound is
D. methane
Polymers are large organic molecules that are made of
d. repeating units
Which of the following groups contain three elements with stable electron configurations?
d. helium, xenon, neon
Typically, atoms gain or lose electrons to achieve
c. a stable electron configuration
In an electron dot diagram, the symbol for an element is used to represent
c. the nucleus and valence electrons.
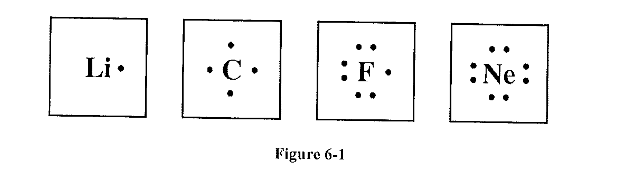
Study the electron dot diagrams for lithium, carbon, fluorine, and neon in Figure 6-1 Choose the statement that correctly identifies the most stable of the elements.
d. Neon is the most stable element because its highest occupied energy level is filled.
The formation of an ionic bond involves the
a. transfer of electrons
Which of the following statements correctly describes the substance with the formula KI?
b. There is a one-to-one ratio of potassium ions to iodide ions.
In the compound MgCI², the subscript 2 indicates that
d. there are two chloride ions for each magnesium ion.
Which of the following compounds does NOT contain molecules?
b. NaCI
When two of the same nonmetal react, they often form a(an)
c. diatomic molecule.
Which of the following formulas represents a compound whose molecules contain a triple bond?
N (triple line) N
In a polar covalent bond,
a. electrons are shared equally between atoms.
Water has a higher boiling point than expected because
d. of the strong attractions between polar water molecules.
The elements most likely to form more than one type of ion are the
a. transition metals.
Fluorine, F, forms a binary ionic compound with lithium, Li. What is the name of this compound?
b. lithium fluoride
Beryllium, Be, and chlorines Cl, form a binary ionic compound with a one-to-two ratio of beryllium ions to
chloride ions. The formula for the compound is
c. BeCl² (subscript)
In the name carbon dioxide, the prefix of the second word indicates that a molecule of carbon dioxide contains
b. two oxygen atoms.
In a periodic table that included electron dot diagrams, in which column would the diagrams contain more dots—Group 2A (the alkaline metals) or Group 6A (the oxygen family)?
6A (oxygen family)
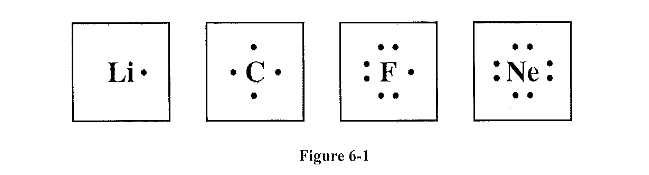
Study the electron dot diagrams in Figure 6-1 Which of the elements are most likely to react and form a compound? What type of compound are they likely to form?
Lithium fluorine (ionic compound)
Two non-metals form a ionic compound 😀
In the binary ionic compound lithium iodide, Lil, which element forms anions?
Iodide
Are covalent bonds more likely to be found in compounds containing both metals and nonmetals or compounds containing only nonmetals?
Only nonmetals is more likely 😁
Suppose a covalent compound has a relatively high boiling point compared to molecules with a similar mass.
Are the molecules in the compound likely to be polar or nonpolar?
The molecules are likely to be polar
In sodium chloride, NaCl, are the cations sodium ions or chloride ions?
Sodium Ions
How do you know that magnesium is the more metallic element in the compound magnesium oxide, MgO?
The compound title begins with “magnesium”. With the naming scheme of compounds, metals/the more metallic elements are placed first in the name.