Ochem Final - Pt. 1
1/19
Earn XP
Description and Tags
Flashcards for reviewing key concepts from Chemistry 341 Exam 1.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
20 Terms
Ascorbic Acid (Vitamin C) Molecular Formula
C7H10O6
Conjugate base of CH3NH2
CH3NH-
Greatest bond angle strain
Cyclopropane
Largest bond dipole moment
C-F
Isobutane
CH3CH(CH3)2
Primary (1°) alkyl group
-CH2CH(CH3)2
Ethers with the formula C4H10O
Three
Bond in (CH3)4NCl
Ionic
Molecular orbitals of ethene (C2H4)
1π+ 1π*
Hybridization of oxygen in methyl alcohol
sp3
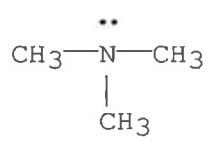
Tertiary amine
N(CH3)3
Strongest acid
HCO2H
IUPAC name of CH3CH2C(CH3)2CH(CH3)CH(CH3)CH2CH3
3,3,4,5-tetramethylheptane
Higher boiling point, CH3CH2OCH3 or CH3CH(OH)CH3?
CH3CH(OH)CH3 because it can form hydrogen bonds.
skeletal structure of cis-1,3-dichlorocyclohexene in a stable chair

Lewis structures for two resonance forms of acetate anions
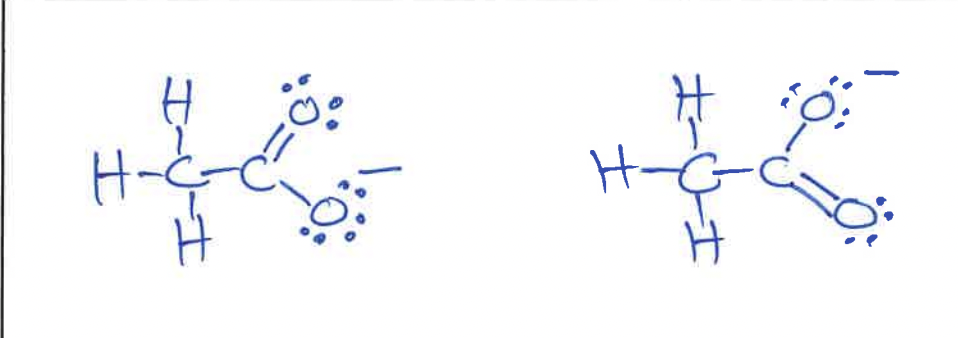
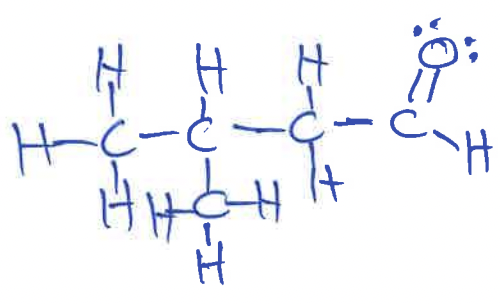
Lewis Structure for (CH3)2CHCH2CO
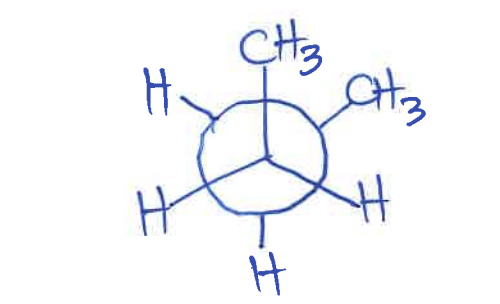
Draw gauche formation as Newman projection