Quality assurance
1/13
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
14 Terms
what is QA
•‘Quality assurance’ is all those planned and systematic actions necessary to provide adequate confidence that the equipment will meet defined standards
•Quality Assurance (QA) programme:
Comprises testing equipment before first use and routinely throughout its life against agreed performance criteria.
Individual Quality Control (QC) tests on all components ensure that the performance of the system remains within acceptable criteria.
what’s the difference between QC and QA
•QA is the overarching system that ensures quality. It includes the planning, policies, procedures, and audits designed to provide confidence that quality requirements will be met.
•QC is a component of QA that focuses on the actual testing and verification activities to ensure that the specified quality standards are being met. It involves the practical measurement, monitoring, and evaluation of equipment performance and processes.
why is QA needed
•It ensures any problems and equipment deterioration are detected.
•It ensures patient and staff safety
•It satisfies legislative requirements - IR(ME)R 17 – so it’s the law!
•It ensures that doses are kept ALARP.
•It ensures optimal image quality and identifies problems.
what are the requirements for equipment quality assurance
•The Medical Physics Expert (MPE) should contribute to the definition and performance of quality assurance for the equipment.
•The QA programme should include all equipment used in connection with the exposure (eg lead aprons, monitors).
•A QA programme should include the provision of appropriate test equipment and its calibration and maintenance
•A QA programme should include procedures for dealing with and recording equipment faults, including when equipment should be taken out of service, when to contact the manufacturer/MPE/RPA, and what tests to perform.
•Records of maintenance, including QA, should be kept for each item of equipment, detailing defects, remedial actions, repairs, and retesting results.
what are the employer’s responsibilities under IRMER
•An Employer who has control over any equipment must implement and maintain a quality assurance programme.
•The Employer must establish and maintain appropriate arrangements for the performance of equipment testing before first use, routinely, and following maintenance that could affect performance.
•Employers should ensure that Operators performing QA tests are adequately trained.
•There should be adequate resources to undertake the tests.
•The Employer should consult their Medical Physics Expert for advice.
how do radiographers act as operators when carrying out QA tests
•Responsibility to report failing tests, carry them out correctly.
•A standard operating procedure (SOP) should govern the QA performed by local staff, detailing test procedures, expected results, action levels, and recording of results.
•This is as important as performing an exam on a patient.
•Every patient that morning could be affected.
what are your responsibilities as operator
•An appropriately trained and entitled individual should have overall responsibility for the QA programme at a local level, providing a clear line of accountability.
•Operators have a responsibility to report any equipment faults or issues that could compromise the safety or accuracy of procedures. This includes failed QC tests.
•Once trained, Operators have a competency to understand the tests, expected results, action levels, and proper recording of findings to identify and address potential equipment problems
What’s the lifecycle of a QA unit?
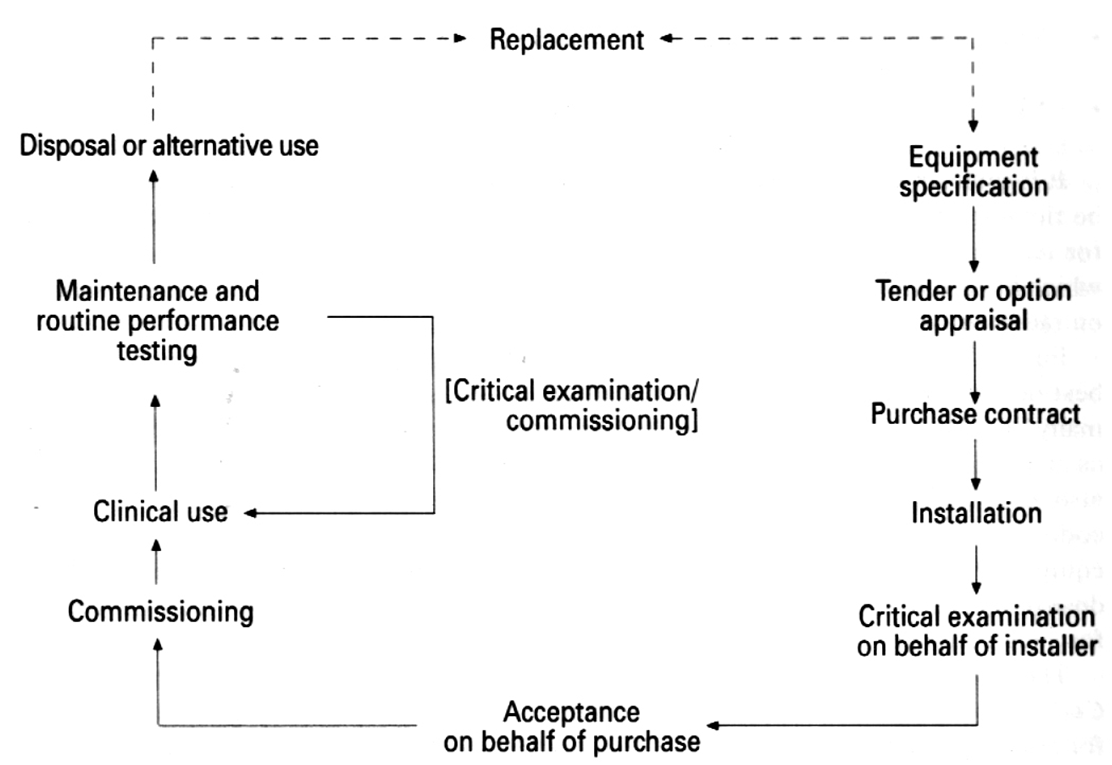
what are the steps necessary from purchase to bring the unit into clinical use
•Critical examination
•Is it safe to use?
•Carried out to ensure basic safety features and warning devices are operating correctly.
•Ensures sufficient protection from exposure to ionising radiation for staff, patients and public.
•Acceptance testing
•Have you bought what you paid for?
•The equipment specification and features are verified by the installer and physicist in this step.
•Checklist is commonly used to compare what was expected from the contract against the actual specification of the equipment.
•Commissioning
•Is it ready for clinical use?
•This step involves a set of tests for all parameters and conditions expected to be used routinely to ensure that the equipment is ready for clinical use.
•The baseline values are set during these tests which allow values obtained in future routine performance tests to be compared against them.
•The baseline values are a measure of how the equipment is performing.
what are routine and reactive performance tests
Routine Performance Tests while in Clinical Use
•Over the equipment's lifetime a set of tests are performed at regular intervals or after maintenance.
•The tests are required to detect any changes in the performance of the equipment.
•These tests will be performed by a variety of staff groups and at different frequencies depending on the complexity and time required for each of the specific tests.
•Corrective action may be required if the results of the tests are unsatisfactory.
Routine performance tests - Level A and Level B tests:
•Level A: frequent, quick and simple tests with pass/fail action and simple test equipment and analysis (Radiographers)
•Level B: less frequent (annually or less frequent), more analytical, sophisticated test equipment, high level of expertise (Medical Physicists)
Reactive performance tests after a major repair
•For example, a collimator replaced.
•Tests done after the engineers have completed work but before clinical use.
what are action levels
Action levels
• Remedial Level:
•Remedial action should be taken, but the unit may continue to be used clinically
•The timescale for carrying out the remedial action and any restrictions on the equipment’s use should be agreed.
Suspension Level:
•The equipment should be taken out of clinical use immediately until the performance is corrected.
•Suspension levels do not exist for tests such as image quality due to them being subjective.
•Level
•Level 2 tests are essential for ‘good practice’, level 2 tests are not essential but considered ‘best practice’.
what are level A tests
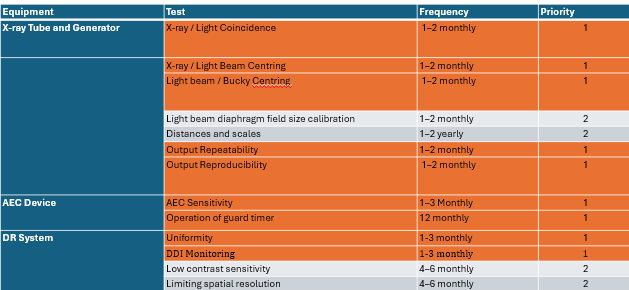
how do you test the guard timer
Place a lead block on the window of the X-ray tube.
Ensure detector is not in the bucky
Select a clinical examination protocol which uses the centre AEC chamber and a kV around 50.
Check that the AEC device terminates at the guard timer setting.
summary
If measurement is outside remedial/suspension:
•Repeat the test to confirm.
•Inform the person responsible for QA.
•Record on the QC form the action to be taken.
•Suspension = equipment not fit for clinical use.
•Remedial = Inform the RPS. Equipment must be repaired by an engineer and QA checked by physics.