Regional Anesthesia - Part 1
1/40
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
41 Terms
Q: What is Point-of-Care Ultrasound (POCUS)?
The use of portable ultrasound by clinicians to guide procedures or assist in diagnosis at the patient’s bedside.
What are the main benefits and limitations of POCUS?
Benefits:
Improves safety and efficacy of regional anesthesia and vascular access;
provides fast, safe, effective, and low‑cost diagnostic support; improves outcomes and reduces costs.
Limitation: Does not replace a formal radiologic examination and must be interpreted within the full clinical context.
Airway, lung, gastric and abdominal evaluations
Guidance of regional and neuraxial techniques
Transthoracic Echocardiography (TTE)
Guidance of central and peripheral vascular access
TTE stands for Transthoracic Echocardiography.
A noninvasive chest-wall ultrasound that visualizes the heart’s structure and function in real time.

Transesophageal Echocardiography (TEE)
Arterial Access
Key contrast (exam favorite)
TTE = Noninvasive, probe on chest wall
TEE = Semi‑invasive, probe in esophagus, better image quality but less immediate
Bladder Scan
» Pain Management (Acute & Chronic)
Airway Management
Urgent decompression of cardiac tamponade
Pain Management (Acute & Chronic)
Needle decompression for pneumothorax
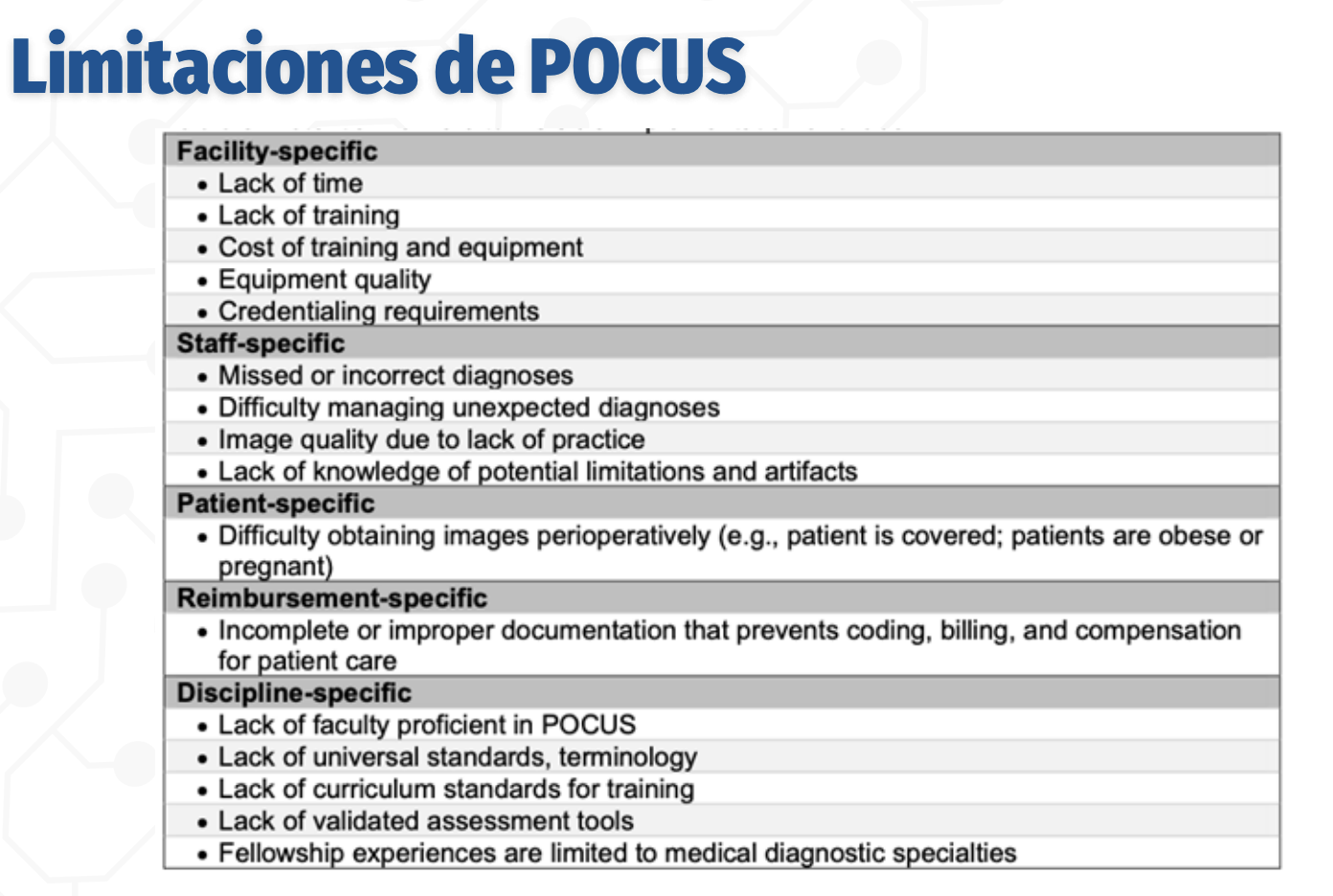
Limitations of POCUS
Facility‑specific: Lack of time and training, high cost of training/equipment, variable equipment quality, credentialing requirements
Staff‑specific: Missed or incorrect diagnoses, difficulty managing unexpected findings, poor image quality due to limited practice, lack of knowledge of artifacts and limitations
Patient‑specific: Difficulty obtaining images perioperatively (e.g., obesity, pregnancy, surgical draping)
Reimbursement‑specific: Incomplete or improper documentation limiting coding, billing, and compensation
Discipline‑specific: Lack of POCUS‑trained faculty, lack of universal standards and terminology, insufficient curriculum standards, lack of validated assessment tools, limited fellowship opportunities
AANA Position on the Use of POCUS
POCUS is a safe, fast, effective, and relatively cost-efficient modality, but its use requires specific training, ongoing education, experience, and a quality assurance program.
CRNAs are well positioned to use POCUS to guide patient care, including: 1. Regional anesthesia and 2. Multimodal pain management, 3. helping reduce or eliminate opioid use and 4. support faster recovery.
The incorporation of regional techniques for preventive analgesia is associated with:
Reduced postoperative pain
Reduced or eliminated opioid use
Decreased incidence of postoperative nausea and vomiting (PONV)
Faster recovery
Shorter length of stay in the post‑anesthesia care unit (PACU)
Shorter time to hospital discharge
Increased patient satisfaction
Reduced incidence of postsurgical pain 3 to 12 months after surgery, decreasing the risk of prolonged opioid use
Regional anesthesia may have a positive long‑term impact on wound healing and immune function
Surgeries with a Higher Incidence of Chronic Pain:
■ Limb amputation
■ Thoracotomy
■ Inguinal herniorrhaphy
■ Abdominal hysterectomy
■ Saphenous vein stripping
■ Open cholecystectomy
■ Nephrectomy
■ Mastectomy

Blunt‑Beveled 25‑Gauge Needle
Used commonly for axillary brachial plexus blocks
Blunt bevel reduces the risk of nerve penetration and intraneural injection
Smaller gauge (25G) → less tissue trauma and patient discomfort
Tactile resistance helps identify tissue planes
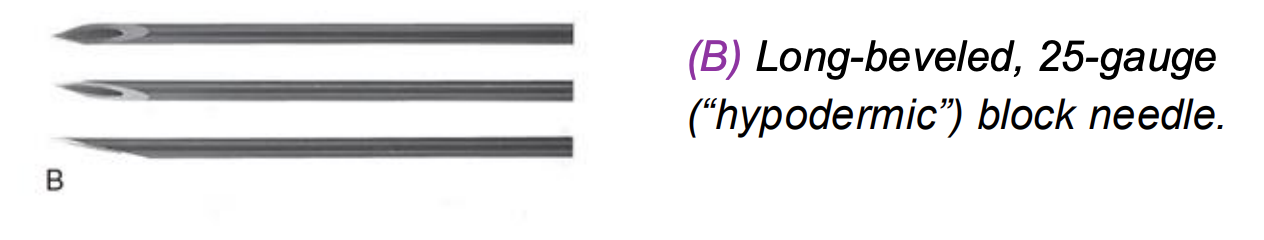
Long-beveled, 25-gauge (“hypodermic”) block needle.
Sharp, long bevel facilitates easy tissue penetration
Historically used for peripheral nerve blocks
Higher risk of nerve injury compared to short or blunt bevels
Less commonly used today due to safety concern
22‑Gauge Ultrasonography “Imaging” Needle
Designed for ultrasound‑guided regional anesthesia
Echogenic coating or textured surface → improved needle visibility
Balance between rigidity and flexibility
Enhances accuracy and safety during nerve blocks
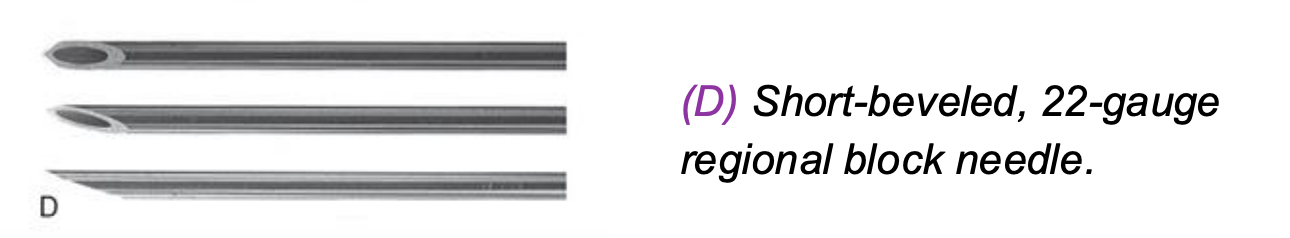
Short‑Beveled 22‑Gauge Needle
Short bevel reduces risk of nerve injury
Better tactile feedback when passing through tissues
Commonly used for peripheral nerve and neuraxial techniques
Provides a safety balance between penetration and control
Ultra‑High‑Yield Exam Pearl
Short or blunt bevels = safer near nerves
Long bevels = higher nerve injury risk

Spinal Needles
Pencil‑point (Sprotte, Whitacre) → ↓ PDPH
Cutting needles (Quincke, Greene) → ↑ PDPH
(A) Sprotte needle
(B) Whitacre needle
(C) Greene needle
(D) Quincke needle
Epidural Needles
(A) Crawford needle
(B) Tuohy needle; the inset shows a winged hub assembly common to winged needles.
(C) Hustead needle.
(D) Curved, 18-gauge epidural needle.
Tuohy = standard epidural needle
Curved tip → directs catheter
Blunt/non‑cutting bevel → ↓ dural puncture risk
Larger gauge = easier catheter passage but ↑ tissue trauma
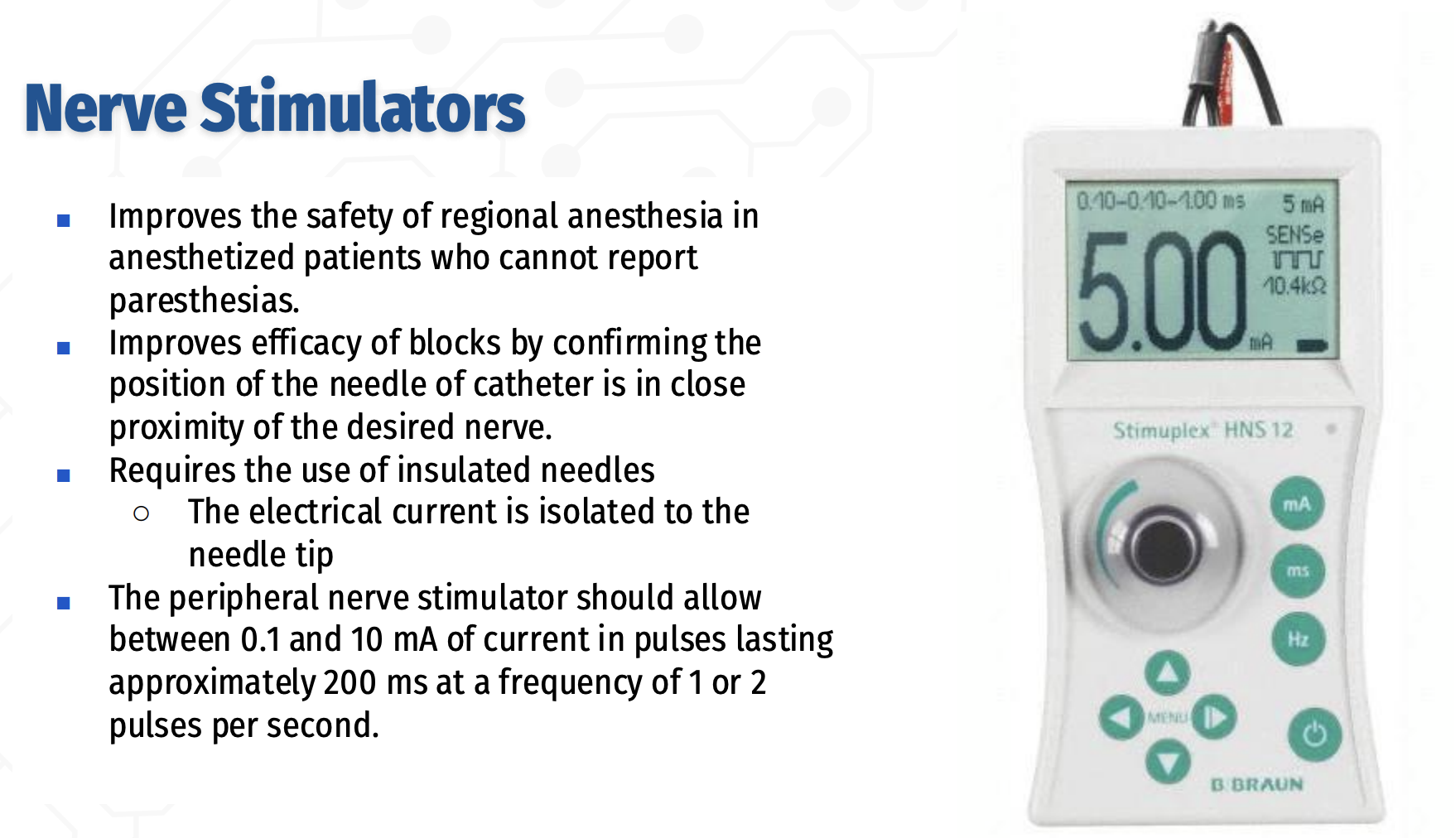
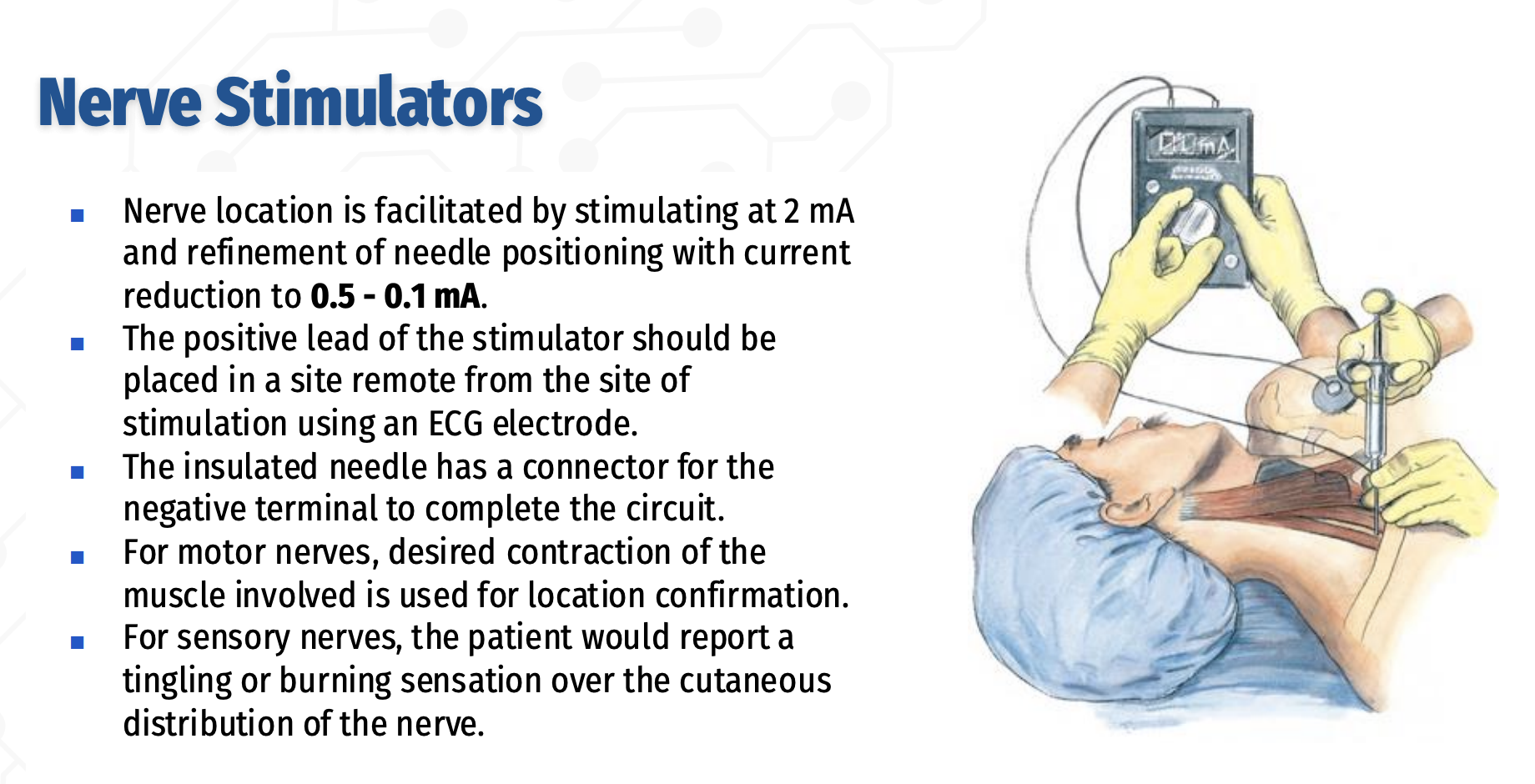
What is the role and key technical requirements of peripheral nerve stimulators in regional anesthesia?
Peripheral nerve stimulators improve the safety and efficacy of regional anesthesia, especially in anesthetized or sedated patients who cannot report paresthesias,
By confirming that the needle or catheter tip is in close proximity to the target nerve.
Their use requires insulated needles to ensure the electrical current is concentrated at the needle tip.
The stimulator should deliver 0.1–10 mA of current in pulses lasting ~200 ms at a frequency of 1–2 pulses per second.
Q: What are the key numerical settings for a peripheral nerve stimulator used in regional anesthesia?
Current range: 0.1–10 mA
Pulse duration: ~200 milliseconds
Stimulation frequency: 1–2 pulses per second
How is a peripheral nerve stimulator used to locate nerves during regional anesthesia?
Nerve location begins with stimulation at 2 mA, followed by refinement of needle position by reducing the current to 0.5–0.1 mA.
The positive electrode is placed remotely using an ECG electrode, and the negative terminal connects to an insulated needle to complete the circuit.
For motor nerves, correct placement is confirmed by the desired muscle contraction; for sensory nerves, the patient reports tingling or burning in the nerve’s cutaneous distribution.
Peripheral Nerve Stimulator – Technique & Confirmation
✅ 2 → 0.5 → 0.1 mA = find, refine, confirm
Start at 2 mA to find the nerve, reduce to 0.5 mA to refine positioning, and avoid injection if responses persist at 0.1–0.2 mA due to intraneural risk.
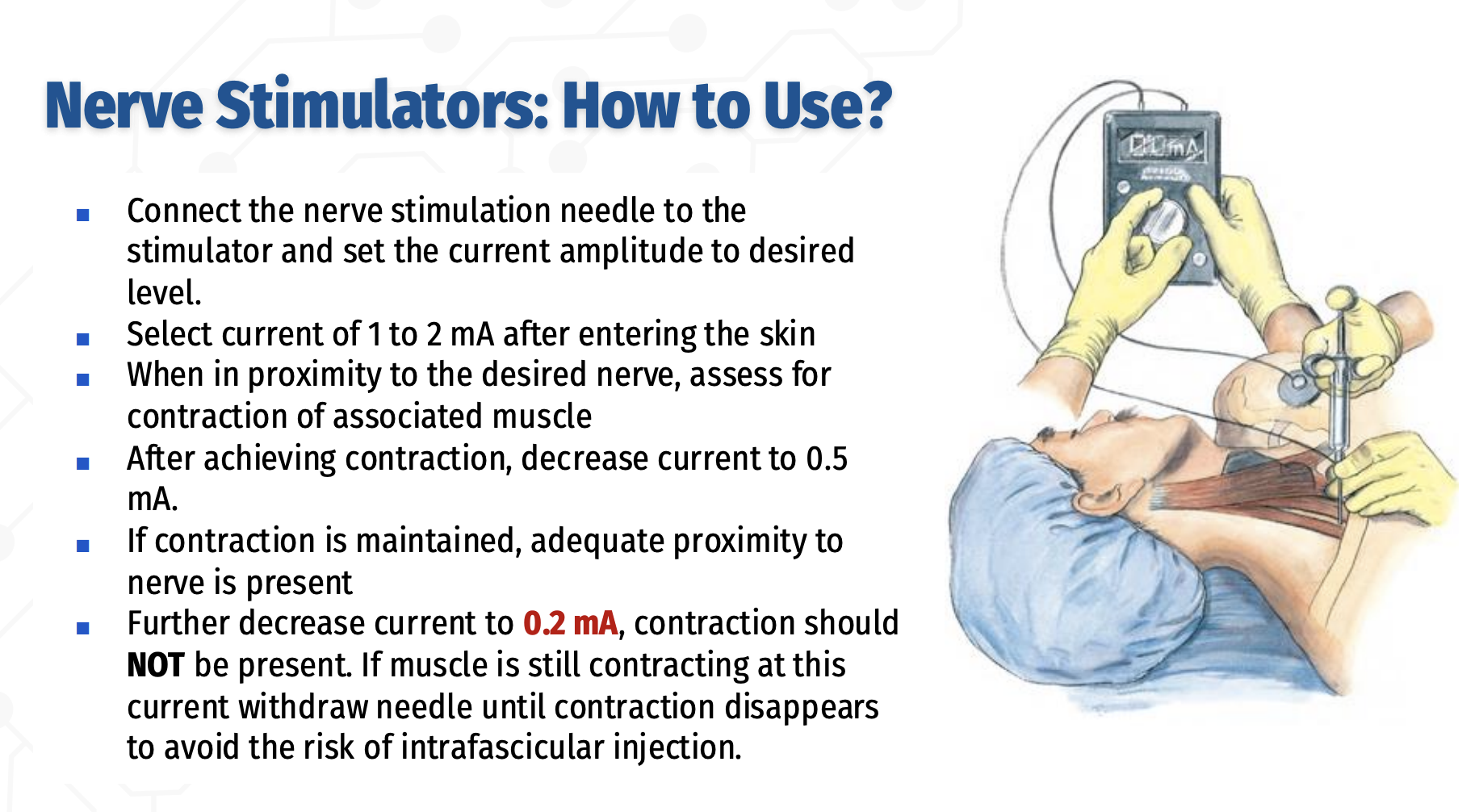
How is a peripheral nerve stimulator used to safely locate a nerve during regional anesthesia?
Connect the insulated nerve‑stimulating needle to the stimulator and set the desired current
After skin penetration, begin stimulation at 1–2 mA to locate the nerve
When near the target nerve, observe for contraction of the associated muscle
Once contraction is obtained, decrease the current to 0.5 mA
Sustained contraction at 0.5 mA confirms adequate needle–nerve proximity
Further reduce the current to 0.2 mA
Muscle contraction should NOT be present
If contraction persists at 0.2 mA, withdraw the needle until it disappears to avoid intrafascicular (intraneural) injection
» ✅ Inject when contraction is present at 0.5 mA
⚠ If contraction persists at ≤0.2 mA → too close → withdraw
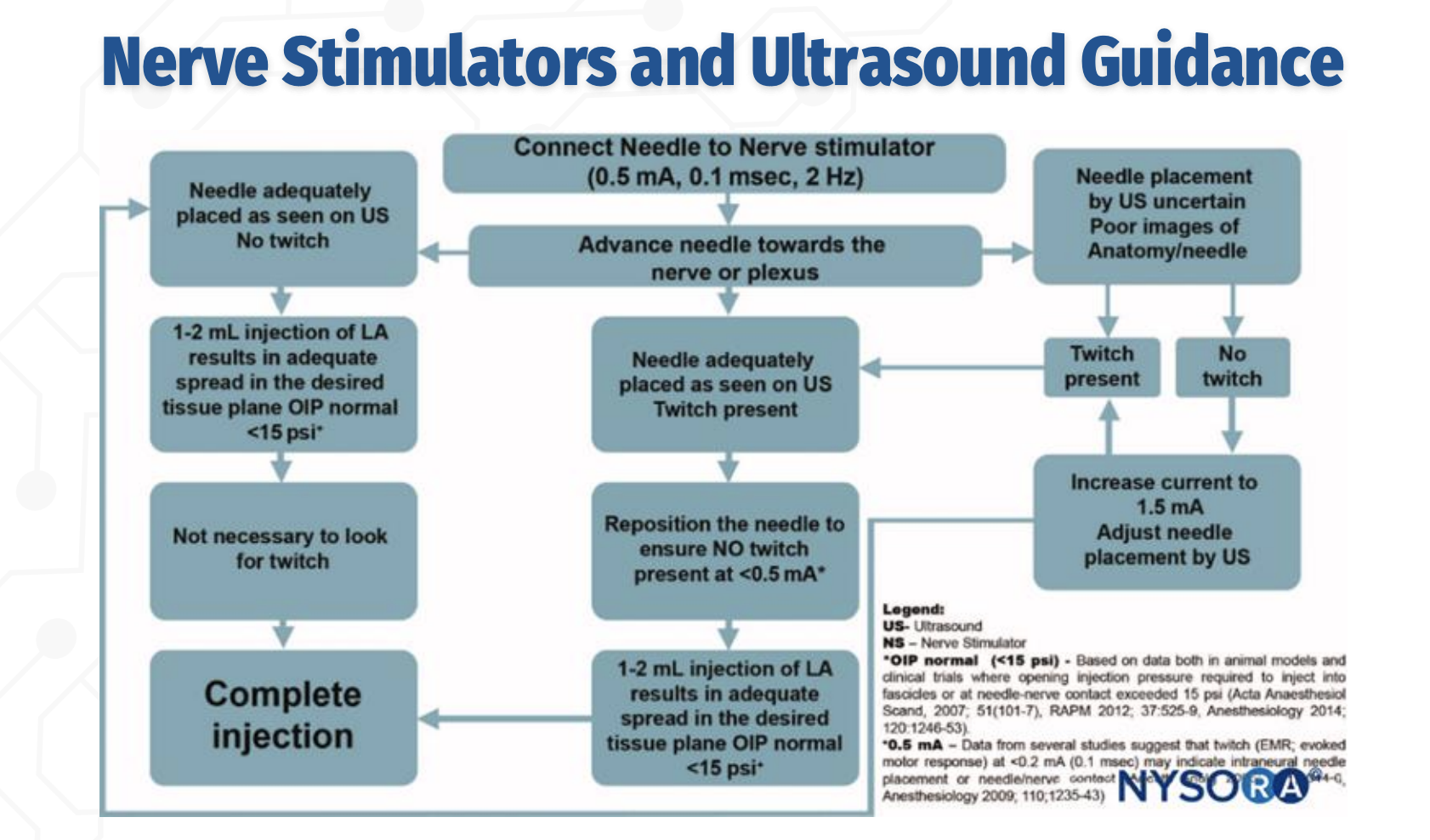
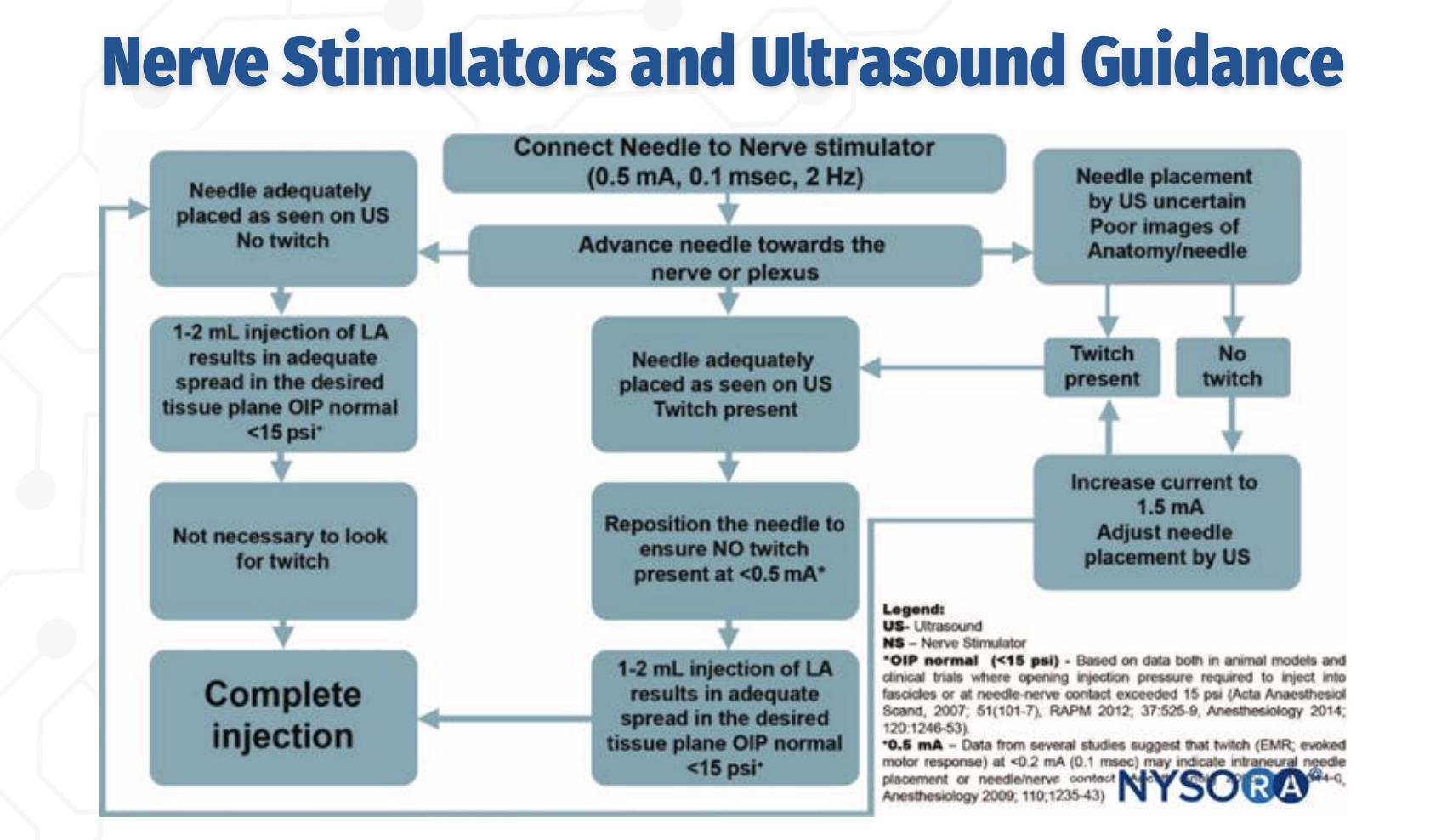
US + Nerve Stimulator: Initial Approach
The needle is advanced toward the nerve under ultrasound guidance while connected to a nerve stimulator set at 0.5 mA, 0.1 ms, 2 Hz. Ultrasound is used to visualize needle–nerve proximity, and nerve stimulation provides functional confirmation, especially when ultrasound images are poor or uncertain.
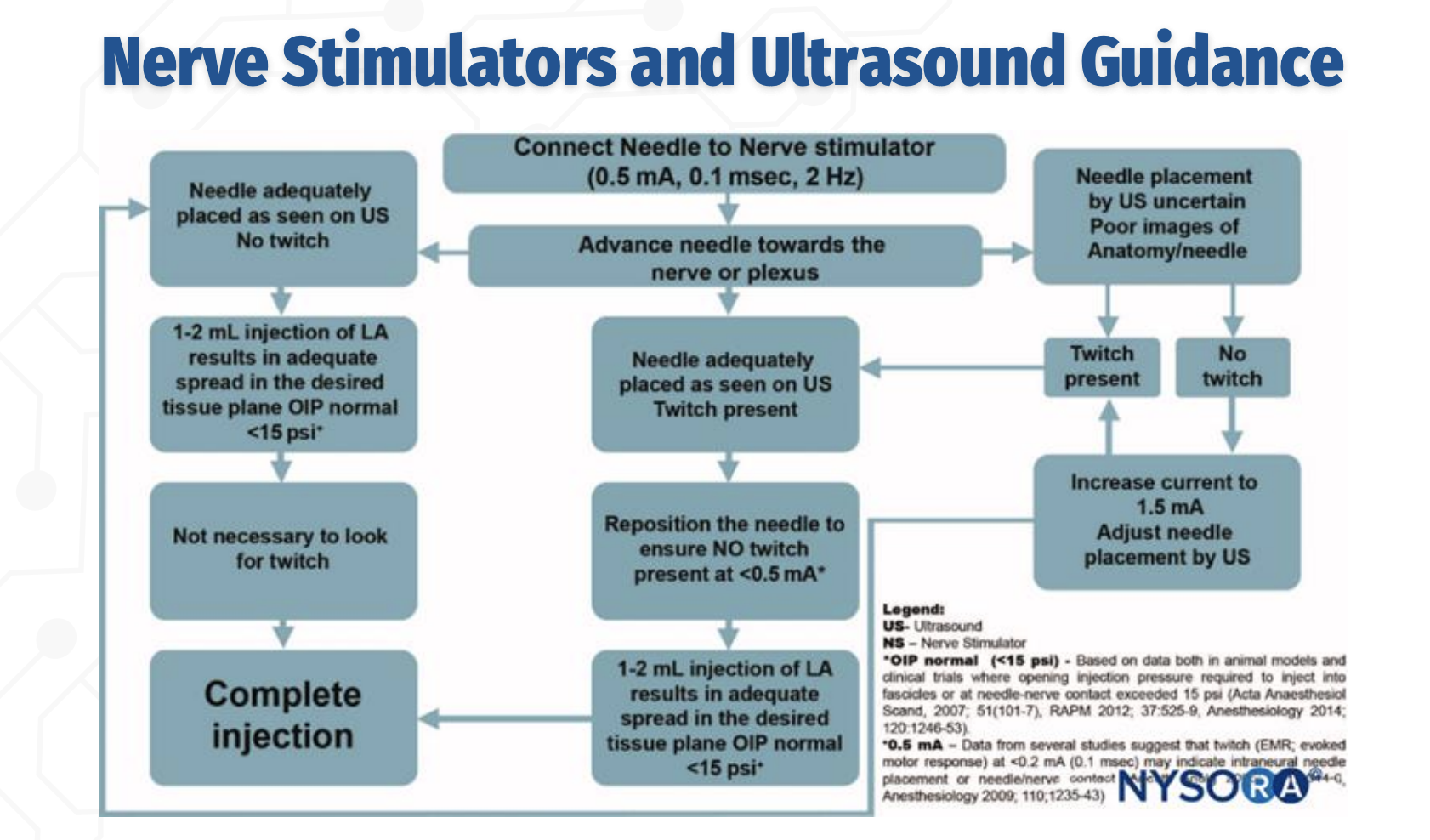
Role of Twitch With Adequate Ultrasound Imaging
Q: If the needle is clearly visualized on ultrasound, is a motor twitch required before injection?
No. If the needle tip is adequately visualized on ultrasound, and a 1–2 mL test injection produces appropriate spread in the correct tissue plane with normal opening injection pressure (<15 psi), the block may proceed without the presence of a twitch.
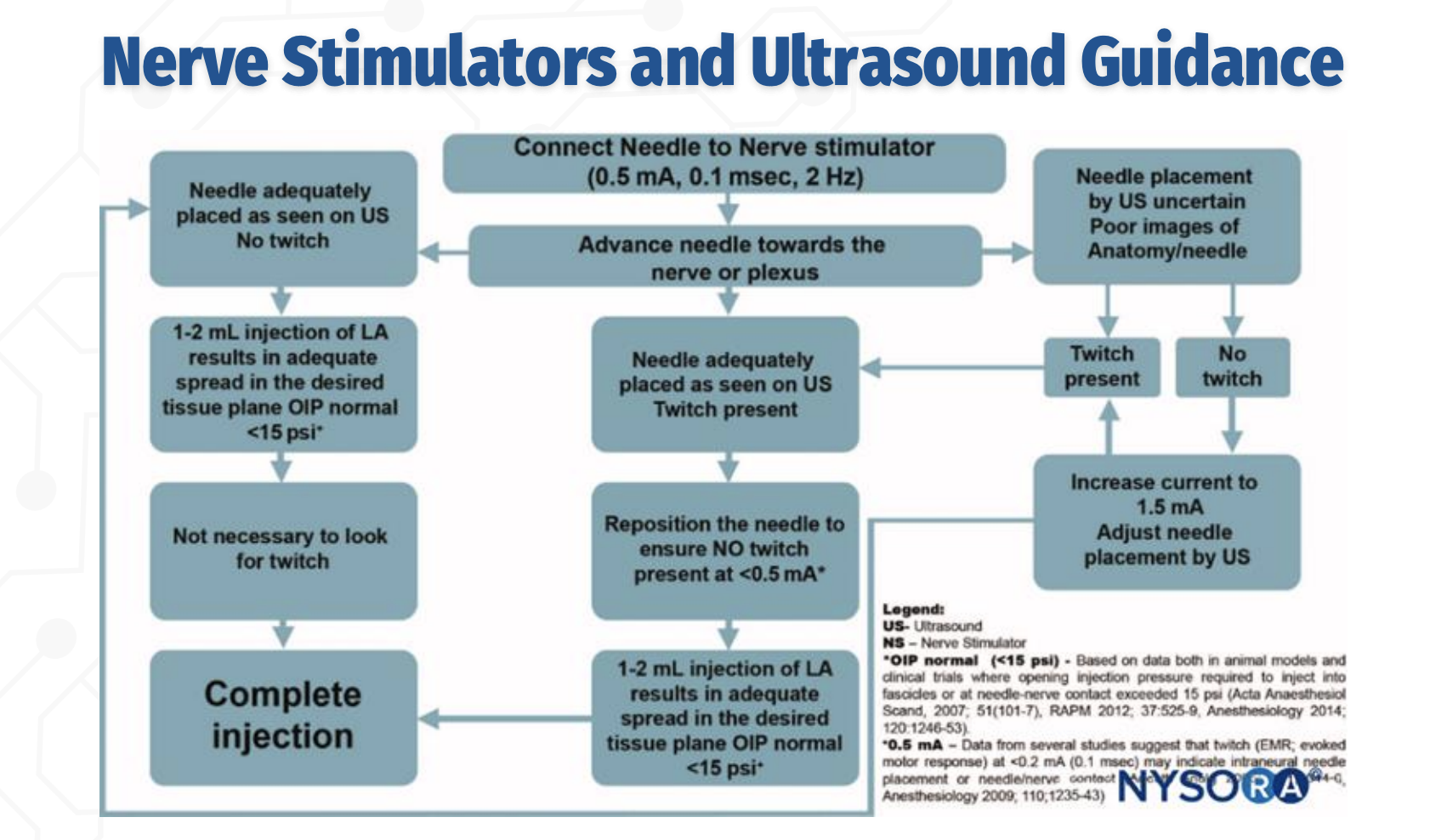
Interpreting Twitch Responses During Combined Guidance
Q: How should twitch responses be interpreted when combining nerve stimulation with ultrasound?
A twitch at 0.5 mA indicates close proximity to the nerve.
The needle should then be adjusted so that no twitch is present at <0.5 mA, as a persistent twitch at very low current suggests needle–nerve contact or intraneural placement, increasing the risk of nerve injury.
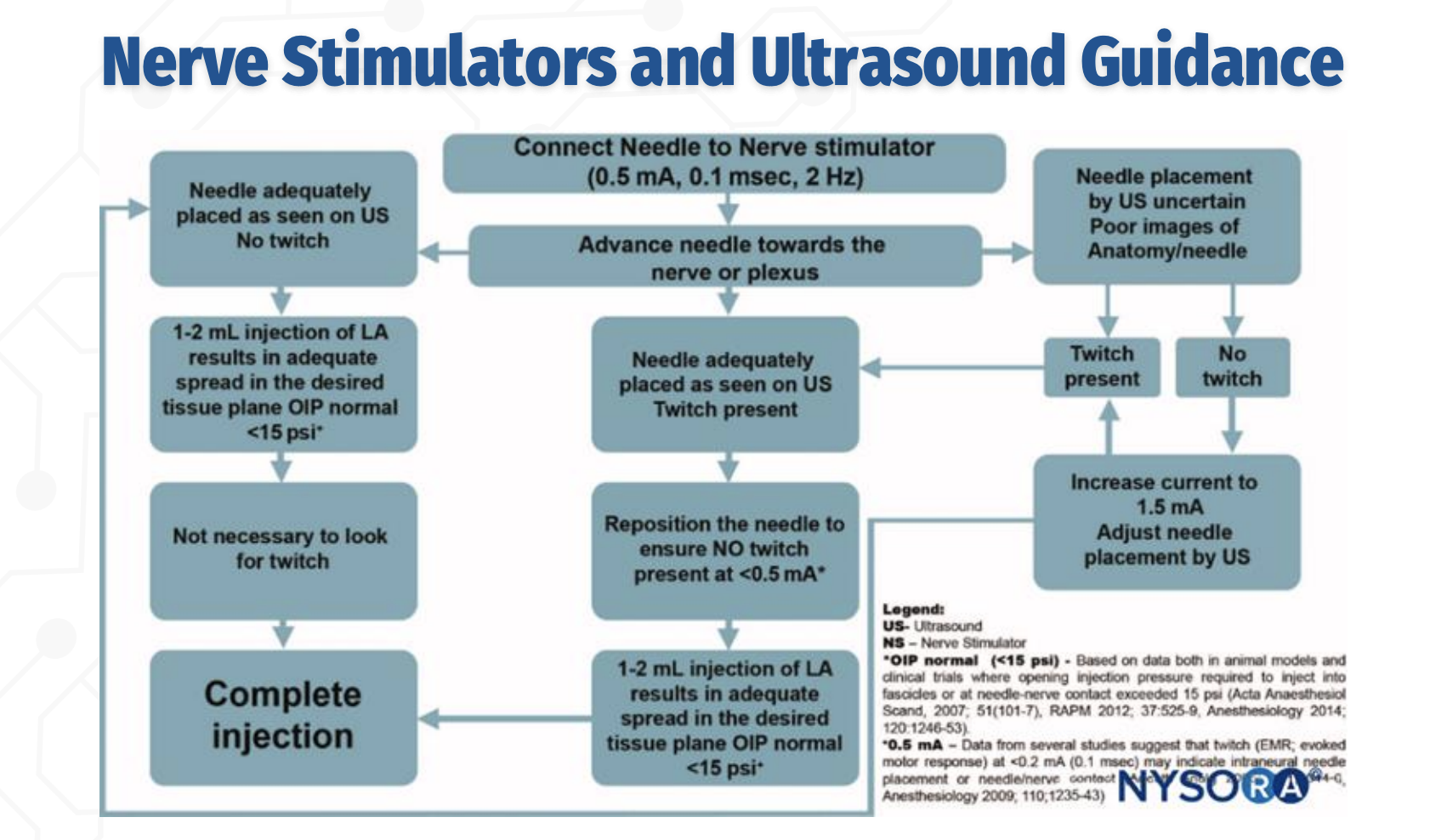
STOP / GO Criteria for Injection (EXAM FAVORITE)
What findings indicate it is safe to proceed with local anesthetic injection during a peripheral nerve block?
✅ Needle tip well visualized on ultrasound
✅ No motor response at ≤0.2–0.3 mA
✅ 1–2 mL test dose shows proper spread in target tissue plane
✅ Opening injection pressure <15 psi
❌ STOP if high pressure, poor spread, or twitch persists at very low current (intraneural risk)
Ultrasound confirms anatomy; nerve stimulation confirms safety — you avoid twitch at very low current, not chase it.
One‑Line Board Pearl
slide 21
Ultrasound confirms anatomy; nerve stimulation confirms safety — you avoid twitch at very low current, not chase it.
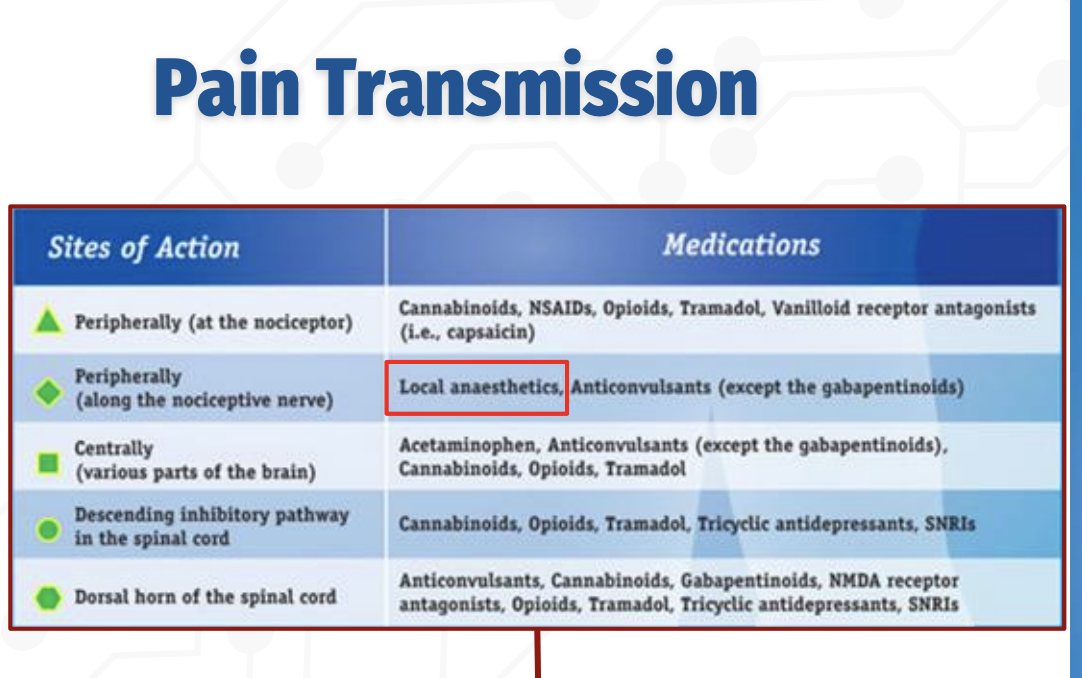
How does pain transmission occur, and where do different analgesic medications act?
Pain transmission can be modulated at multiple levels of the nervous system, and different drug classes act at specific sites:
Peripherally (at the nociceptor):
NSAIDs, opioids, tramadol, cannabinoids, and vanilloid receptor agents (e.g., capsaicin) reduce pain signaling at the site of injury.Peripherally (along the nociceptive nerve):
Local anesthetics block voltage‑gated sodium channels, preventing action potential propagation along the nerve.Centrally (brain):
Acetaminophen, opioids, tramadol, cannabinoids, and anticonvulsants alter pain perception.Descending inhibitory pathways (spinal cord):
Opioids, tramadol, cannabinoids, TCAs, and SNRIs enhance inhibitory control of pain transmission.Dorsal horn of the spinal cord:
Anticonvulsants (including gabapentinoids), NMDA antagonists, opioids, TCAs, SNRIs, and cannabinoids inhibit synaptic transmission of pain signals.
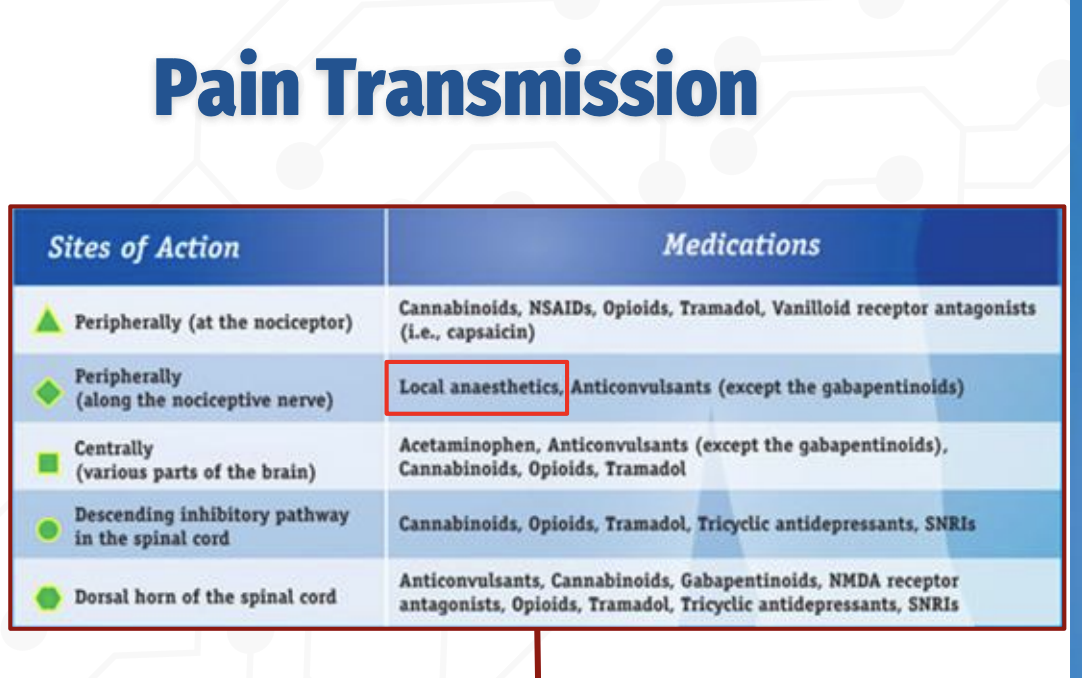
Peripheral vs Central Sites of Pain Modulation
Q: What drugs act peripherally vs centrally in the pain pathway?
Peripheral (nociceptor): NSAIDs, cannabinoids, opioids, tramadol, capsaicin
Peripheral (along nerve): Local anesthetics (block Na⁺ channels)
Central (brain): Acetaminophen, opioids, tramadol, cannabinoids, anticonvulsants
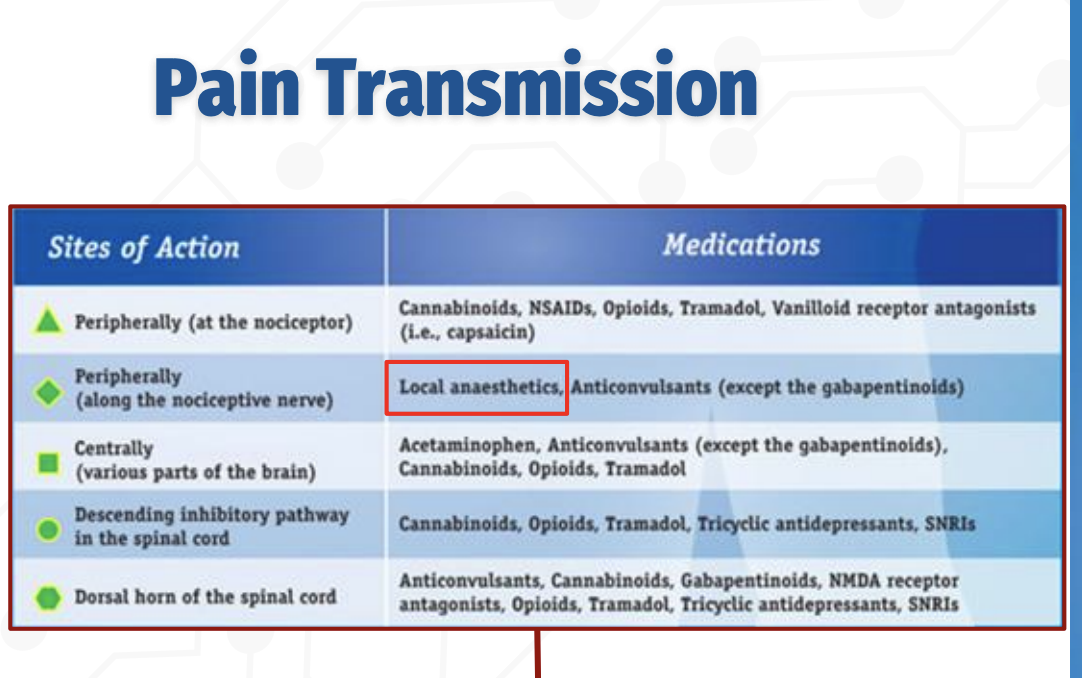
Q: What drug classes act on the spinal cord to reduce pain transmission?
Spinal Cord Modulation of Pain
Descending inhibitory pathways: Opioids, tramadol, cannabinoids, TCAs, SNRIs
Dorsal horn synapses: Anticonvulsants (gabapentinoids), NMDA antagonists, opioids, TCAs, SNRIs, cannabinoids
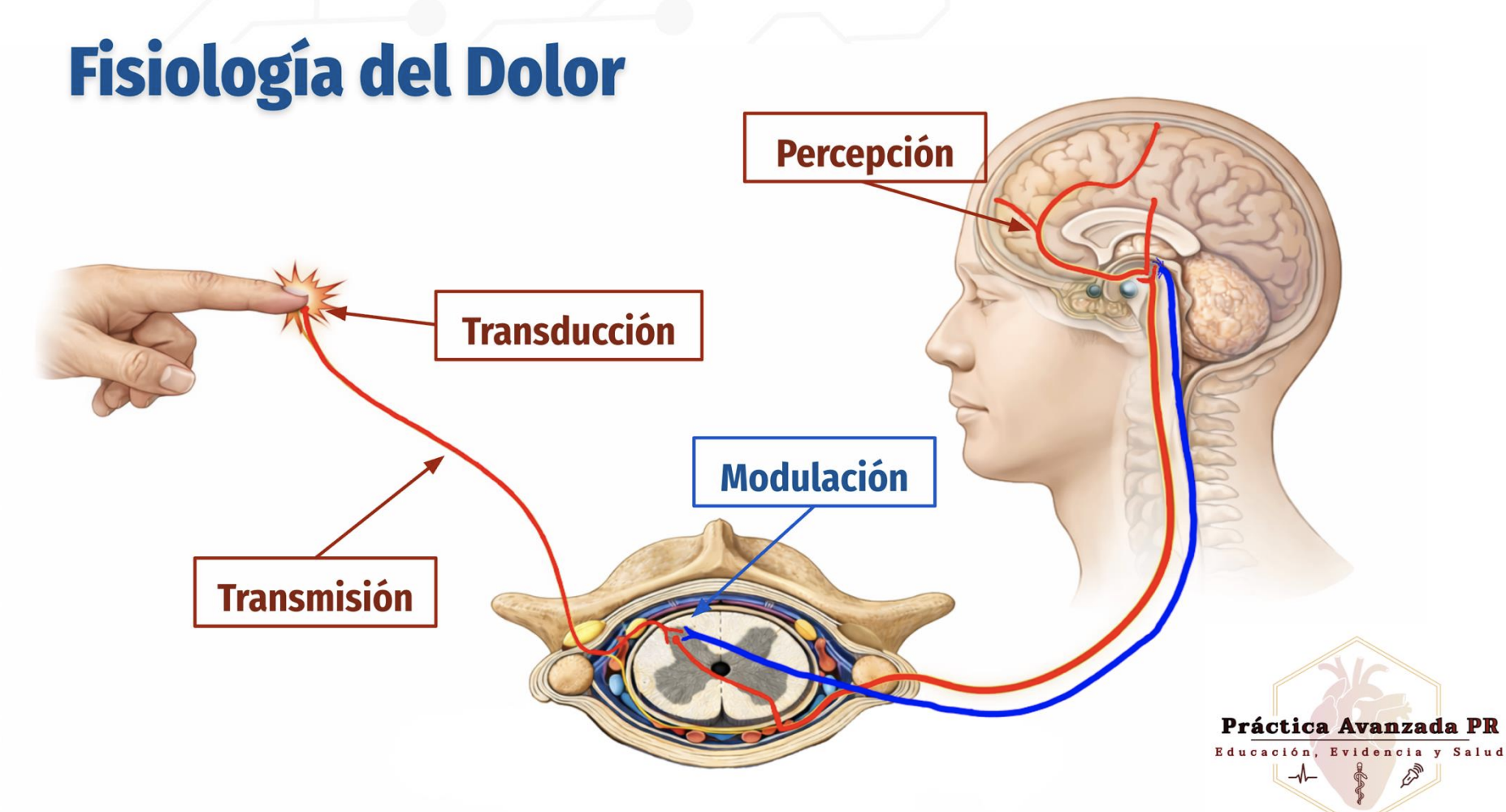
Physiology of Pain
4 stages of pain
Transduction: Conversion of noxious stimuli into nerve impulses at the site of injury
Transmission: Propagation of pain signals from peripheral nerves to the spinal cord and brain
Perception: Conscious recognition and interpretation of pain in the brain
Modulation: Alteration of pain signals via descending inhibitory pathways, primarily in the spinal cord

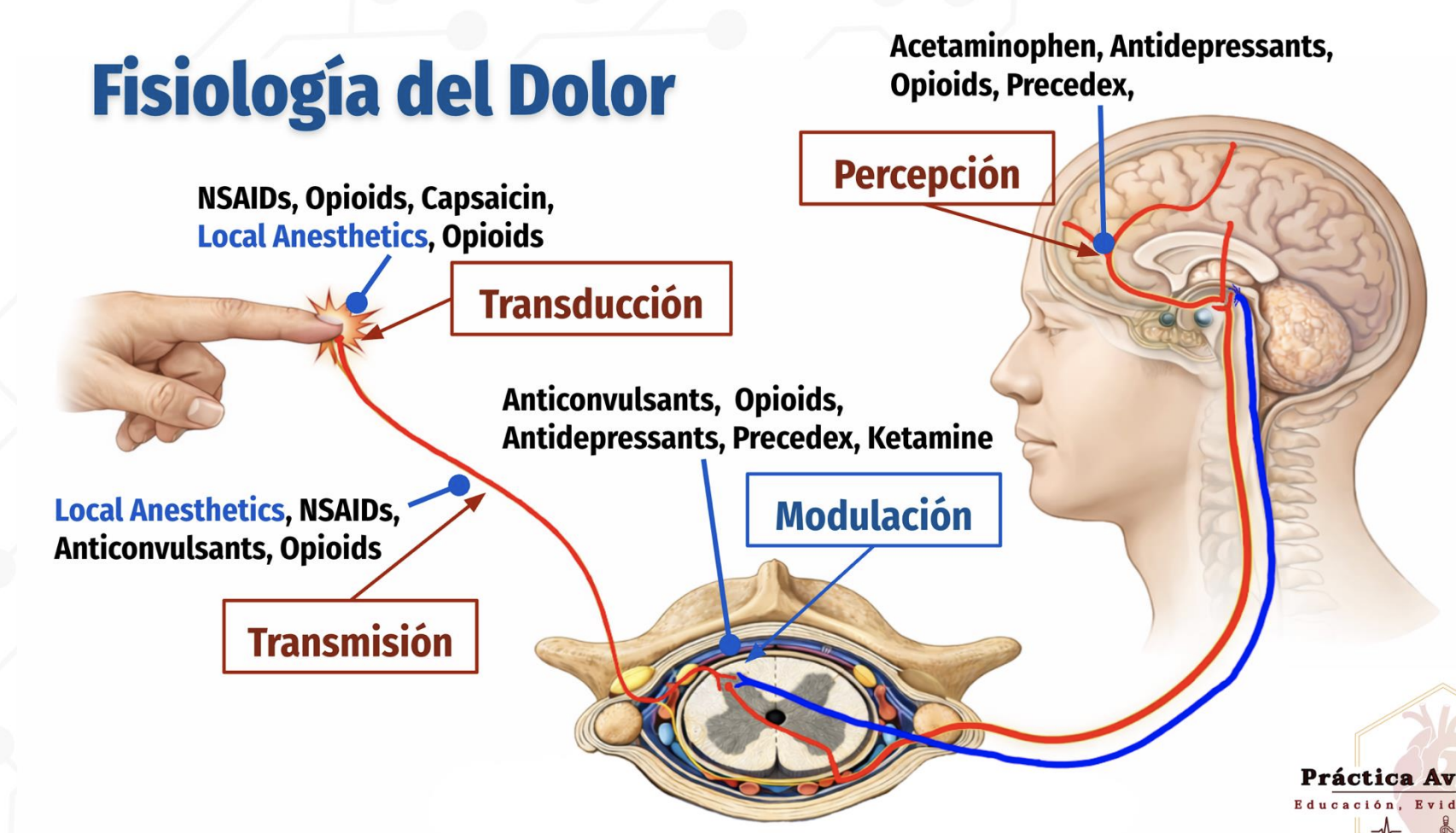
Pain physiology
four stages of pain physiology and the major drug classes acting at each stage
Transduction: Peripheral conversion of stimulus → pain signal
NSAIDs, opioids, capsaicin, local anestheticsTransmission: Pain signal travels along nerves and spinal cord
Local anesthetics, NSAIDs, opioids, anticonvulsantsPerception: Brain recognizes/experiences pain
Acetaminophen, antidepressants, opioids, PrecedexModulation: Descending inhibition of pain in spinal cord
Anticonvulsants, opioids, antidepressants, Precedex, ketamine
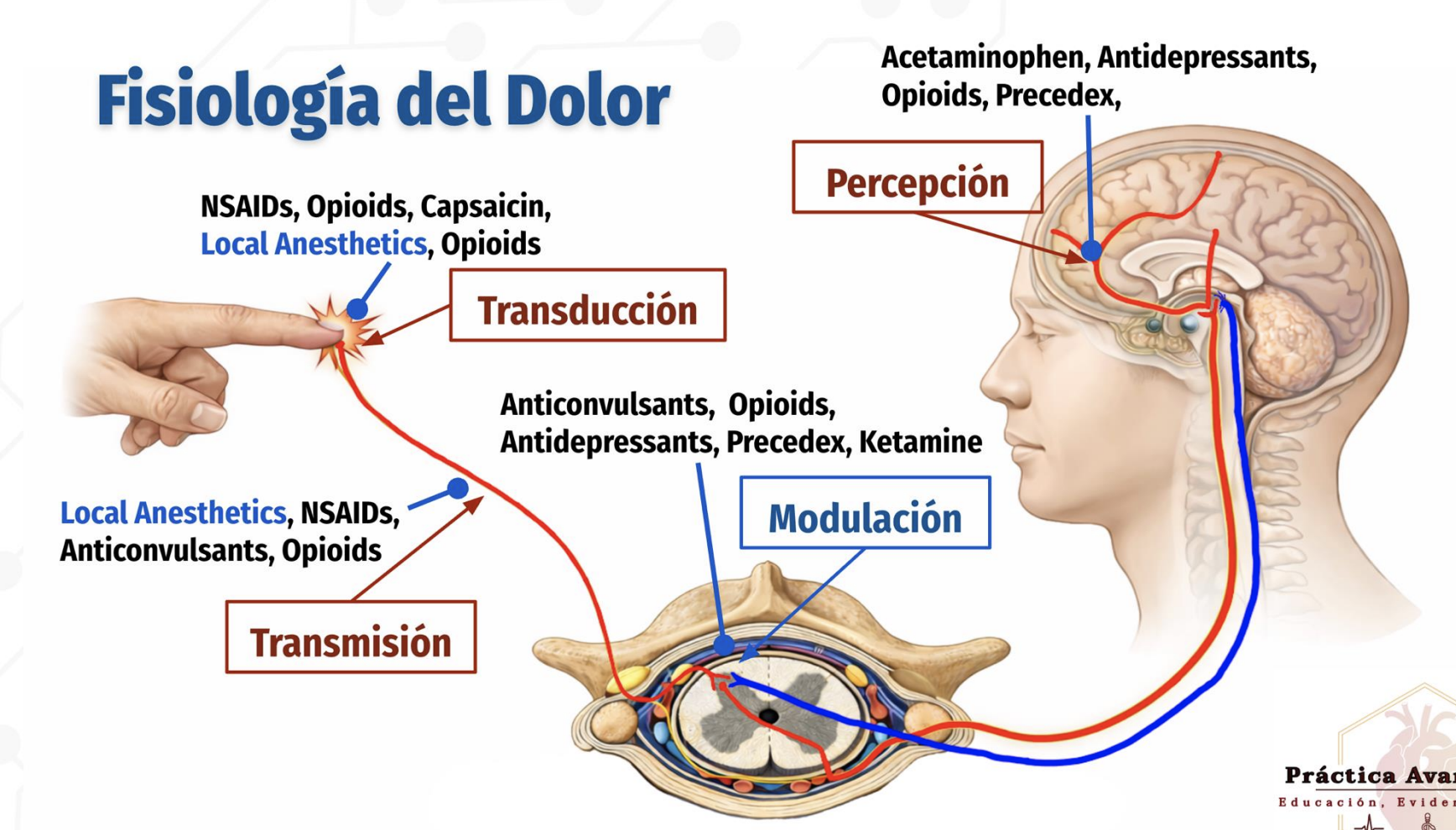
Where Local Anesthetics Work
Local anesthetics act at two key stages:
Transduction: Block nociceptor activation
Transmission: Block sodium channels along the peripheral nerve to stop action potential propagation
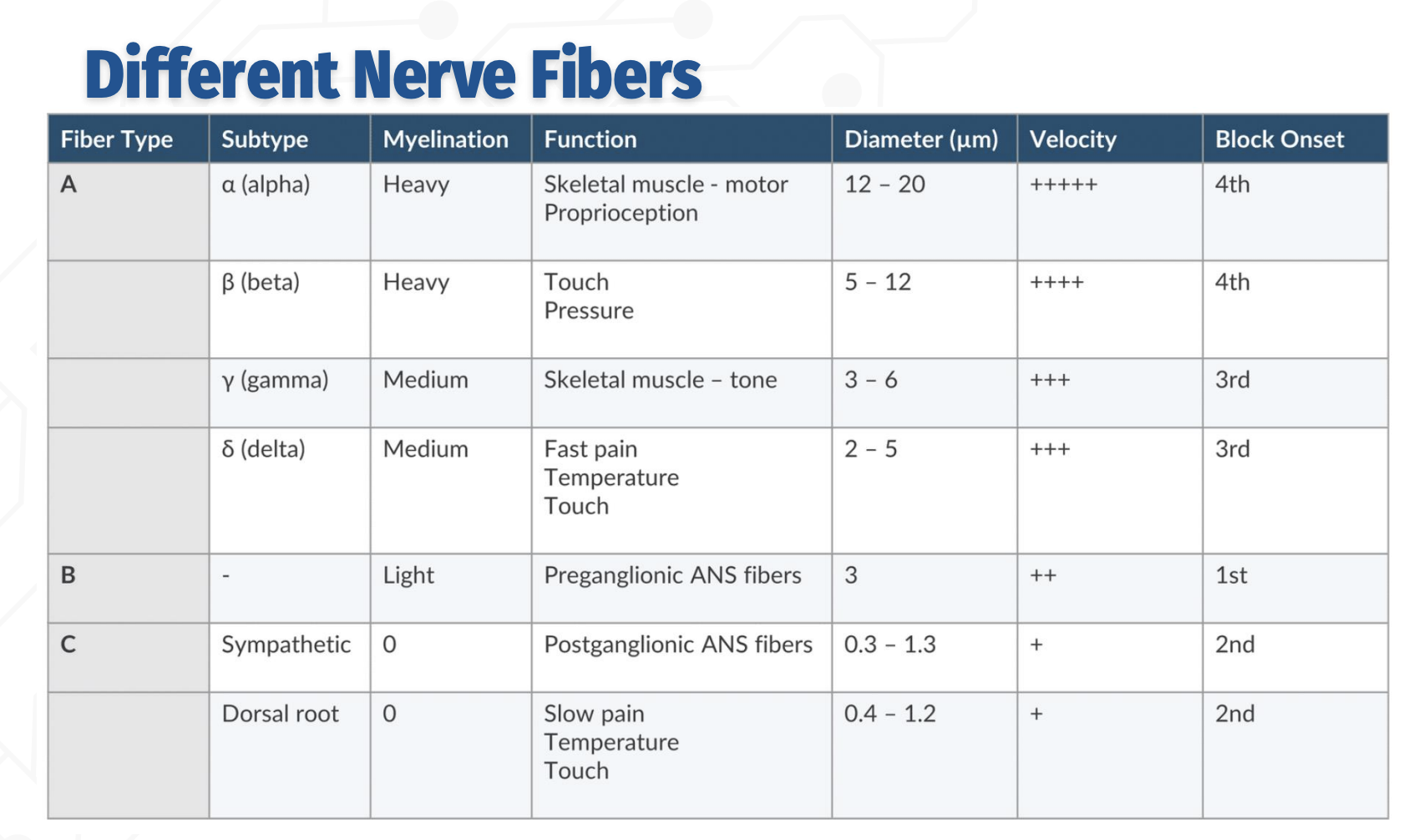
Order of block onset (from most to least sensitive):
B → C → Aδ/γ → Aβ → Aα
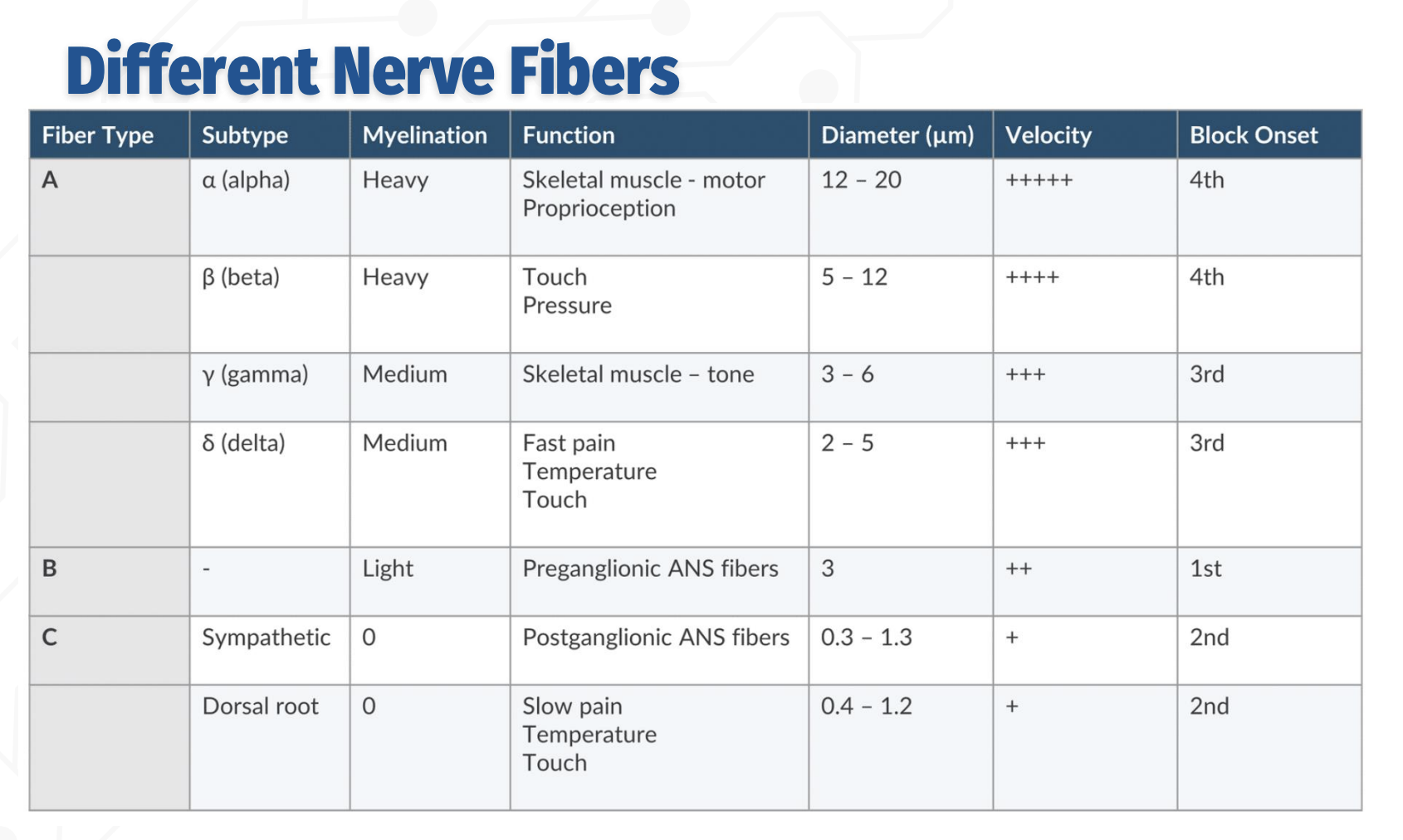
Order of nerve blocks - in detail
C fibers (unmyelinated):
Function: Slow pain, temperature, touch; postganglionic sympathetic fibers
Smallest diameter (0.3–1.3 µm), slowest conduction
Blocked 2nd
B fibers (lightly myelinated):
Function: Preganglionic autonomic fibers
Diameter ~3 µm, moderate conduction velocity
Blocked 1st (most sensitive)
A fibers (myelinated):
δ (delta): Fast pain, temp, touch — 2–5 µm → 3rd block onset
γ (gamma): Muscle tone — 3–6 µm → 3rd block onset
β (beta): Touch & pressure — 5–12 µm → 4th block onset
α (alpha): Motor & proprioception — 12–20 µm, fastest conduction → 4th block onset (last)
Differential Blockade
refers to the fact that different nerve fibers have different sensitivities to local anesthetics, largely based on tissue characteristics and fiber diameter. Smaller fibers require less local anesthetic to be blocked than larger fibers, even if their functions are similar.
Differential Blockade
Order of Block Onset:
Sympathetic fibers → first to be blocked
Acute pain, temperature, and pressure sensation → second
Motor function → last
» A hallmark of early blockade is sympathectomy, which causes cutaneous vasodilation and increased skin temperature in the blocked region