6.2 Radioactivity
1/18
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
19 Terms
Detecting radioactivity: SPARK COUNTER
> f__ w__ stretched below piece of metal g__
> high v__ put between, enough until almost s__
> w/ t__, put r__ near gauze
> rays from it i__ air = better c__ = s__
fine wire, gauze, voltage, sparking, tweezers, radium, ionise, conductor, spark
Detecting radioactivity: PHOTOGRAPHIC FILM
> r__ will b__ photographic f__ unless smth big covers e.g. x-ray
radioactivity, blacken, film
Detecting radioactivity: Geiger–Müller tube
> m__ tube w/ thin w__ in centre, has g__ at low p__
> like spark counter but less V (no spark), instead c__ made
> amplified & passed to scaler/ratemeter
metal, wire, gas, pressure, current
Nuclear Emissions
> r__ emissions are s__ & r__ in direction
> u__ nucleuses emit r__ to be more s__
radioactive, spontaneous, random, unstable, radiation, stable
42__ or __α
> shown w/ __ nucleus
> __ve charge
> __ ionising
> __ penetration
> stopped by t__ p__/s__
He, 4 2, He, +, high, weak, thin paper, skin
0-1__ or __β-
> shown w/ __-
> __ve charge
> __ ionising
> __ penetration
> stopped by a__
e, 0 -1, e, -, mild, mild, aluminium
γ
> shown w/ γ
> __ charge
> __ ionising
> __ penetration
> stopped by thick l__/c__
0, weak, high, lead, concrete
List the radiations, top to bottom
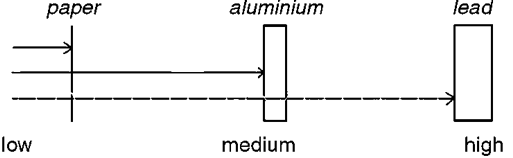
alpha, beta, gamma
beta has more d__ than alpha bc of s__ m__
gamma has passes s__ thru as it has no c__
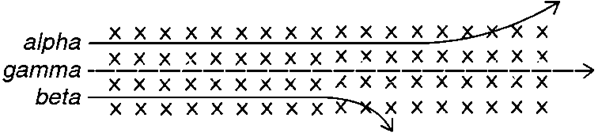
deflection, smaller mass, straight, charge
w/ alpha & beta decay, nucleus changes to diff e__
element
α-emissions
> will lose _p & 2_
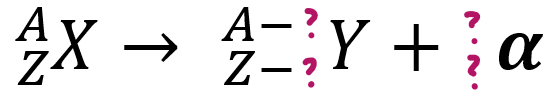
2, n, 4, 2, 4, 2
β-emissions
> fast moving e- e__ from n__
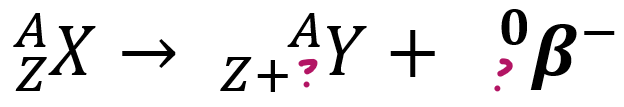
ejected, nucleus, 1, -1
γ-emissions
> high e__ EM waves
> usually part of alpha/beta decay
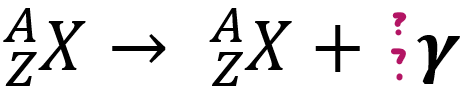
energy, 0, 0
Effect of alpha/beta decay on the nucleus
> ↓# of excess n__
> ↑nucleus s__
neutrons, stability
Isotopes can be radioactive bc:
Ø E__ n__ in nucleus
AND/OR
Ø Nucleus too h__
excess neutrons, heavy
Half-life
time taken for 1/2 the particles in a sample to decay
Which letter shows 1 half-life
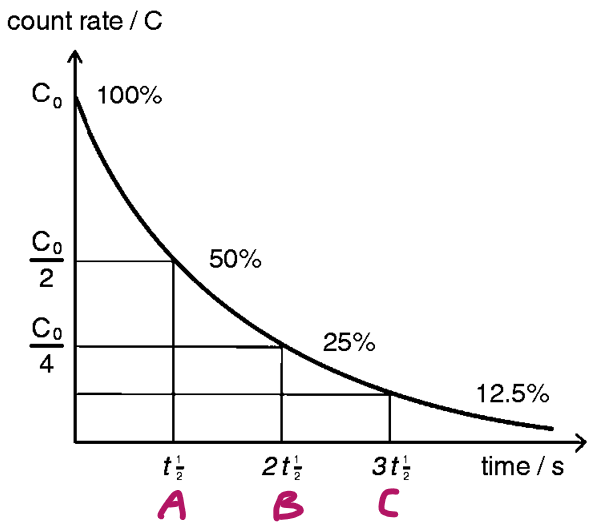
A
Fission reactions
Ø Large n__ splits → 2 lighter nuclei & e__
> neutrons emitted strike other U nuclei = c__
> f__ reactors use U-235 as f__
nucleus, energy, chain, fission, fuel
Safety precautions
Ø i__ radiation = m__, burns, cell d__
1. Store in l__ container
2. Minimise e__; wear lead s__, etc
3. Handle w/ long t__
ionising, mutation, death, lead, exposure, shield, tongs