How the Atomic Model Changed Over Time (with Experiments)
1/4
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
5 Terms
1.John Dalton (1803) – Solid Sphere Model
Idea: Atoms are tiny, solid balls. Each element has its own kind of atom.
Experiment: Dalton didn't do one single experiment for this model, but he based his ideas on chemical reactions and the laws of conservation of mass and definite proportions.

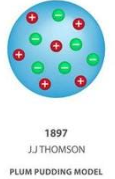
2.J.J. Thomson (1897) – Plum Pudding Model
Discovery: The electron, a tiny negatively charged particle.
Experiment: Cathode Ray Tube Experiment
He passed electricity through gases in a sealed tube.
A beam was attracted to a positive plate, showing the beam was negative (electrons).
Model: A ball of positive charge with negative electrons spread through it like plums in a pudding
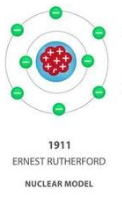
3.Ernest Rutherford (1909) – Nuclear Model
Discovery: The atom has a tiny, dense, positive nucleus.
Experiment: Rutherford Scattering Experiment
Alpha particles were fired at thin gold foil.
Most went straight through, but some were deflected or bounced back.
Showed that most of the atom is empty space, with mass concentrated in the nucleus.
4. Niels Bohr (1913) – Shell Model
Idea: Electrons move in fixed orbits (shells) around the nucleus.
Experiment: Bohr studied the light spectrum (especially of hydrogen).
He found that atoms give off light in specific patterns.
This matched the idea of electrons jumping between energy levels and releasing energy

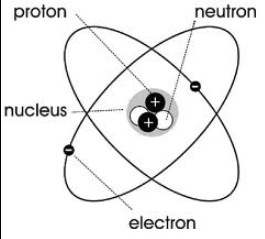
5. James Chadwick (1932) – Discovery of the Neutron
Discovery: The neutron, a particle in the nucleus with no charge.
Experiment: Chadwick used radiation experiments and found that some particles had mass but no charge.
Helped explain why atoms had more mass than just protons could account for.
Importance: Completed the picture of the atom’s nucleus (protons + neutrons).