CHEM241 FINAL (All Units, Cumulative)
1/233
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
234 Terms
List the times of EM radiation from lowest to highest energy.
Radiowave, microwave, infrared, visible, UV, x-ray, gamma.
Wavelengths for all the different colors in the visible spectrum.
400-500nm: violet, blue
500-600nm: green, yellow, orange @ 600
600-700nm: red
What type of transitions do radiowaves cause?
Nuclear spin, some rotational transitions.
What type of transitions do microwaves cause?
Rotational transitions.
What type of transitions does IR cause?
Vibrational transitions.
What type of transitions does visible cause?
Electronic transitions (excitations).
What type of transitions does UV cause?
Valence electron transitions.
What type of transitions do x-rays cause?
Core electron transitions.
What is Beer’s Law? Be able to describe the relationship between absorbance and transmittance.
A = molar absorptivity x b x C
A = -log(T), where T = P/Po
What does molar absorptivity vary with for a molecule?
Wavelength. Absorbance and molar absorptivity changes based on the wavelength of light being transmitted.
What wavelength in a compound’s absorbance spectrum is best to choose if doing a study of the compound’s concentration in various solutions?
Wavelength of maximum absorbance so that you get the most signal.
Under what circumstances does Beer’s Law fail? What is the ideal range of absorbance?
Super high concentrations (very high absorbance) will result in non-linearity, stray light interferences. Beer’s Law also only works for monochromatic wavelengths. Ideal range of absorbance is 0.4-0.9. Can fix via dilutions.
Describe sources of error which dominate spectrophotometric measurements at low and high absorbances.
Low absorbance: instrumental noise becomes a lot more significant, causes baseline instability.
High absorbance: detector saturation is so high that you can’t tell between small differences in transmittance, will cause non-linearity in regards to Beer’s.
Describe what happens when a molecule absorbs light.
Molecule is excited from the ground state to the excited state
Depending on the energy of the photons absorbed, vib/rot/bond transitions may occur.
The molecule will eventually relax with non-radiative relaxations or luminescence (emission forms)
While returning to a ground state, the molecule may release heat via non-radiative relaxation and IC/ISC.
Discuss the difference between electronic excited states, vibrational excited states, and rotational excited states.
Electronic excited states: e- moves from low to high energy orbitals. This requires more energy than vibrational or rotational transitions, and usually involves visible, maybe UV light.
Vibrational excited states: Stretching and bending of atomic bonds. Less energy than electronic excitation, more than rotational. Typically IR.
Rotational excited states: Rotation of a whole molecule around its axes. Least energy required, typically microwave.
What is the difference between a singlet and triplet excited state?
Singlet (So and S1) → 2(0) + 1 = 1
Fluorescence occurs here
Triplet (T1) → 2(1) + 1 = 3
Phosphorescence occurs here
What is radiationless decay and how does it happen?
Molecule in its excited state returns to a lower energy state without emitting photons or light. It loses energy internally, giving off heat → hence, non-radiative.
What are the differences between fluorescence and phosphorescence?
Fluorescence = S1 → So transition
10-8 - 10-4s range (ns to ms)
Phosphorescence = T1 → So transition
10-4 - 102s range (ms to min)
Takes longer because involves a spin flip between triplet and singlet states
What does luminescence mean?
Light emission by a molecule or atom after it absorbs energy from a non-heat source (fluoro and phospho are types of luminescence).
What are the differences between the absorption and emission spectra for a compound?
Absorption spectra show wavelengths of light absorbed by a molecule/atom; dark lines or dips at certain wavelengths. Energy in.
Emission spectra show wavelengths of light emitted as it returns to a lower energy state; bright lines or peaks at certain wavelengths. Energy out.
Wavelengths of absorbance and emission are similar, but not exactly the same (emission marginally higher). Emission loses some energy (to things like vibrational relaxation) before emission and return to ground state (think about Jablonski).
Be able to draw the instrumental layout for both an absorption spectrophotometer and for an emission spectrophotometer. What are the similarities and critical differences. Draw it out.
Refer to notes for correct diagrams.
Both use…
monochromator to isolate a certain wavelength
detector
display
Critical differences…
Light sources: absorbance uses continuous excitation, emission may not even need excitation source.
Monochromator location: absorbance is before the sample, emission is after the sample.
Measures? absorbance measures light going through the sample, emission is when the sample is excited and the light is measured through it.
Signal depends on? absorbance is how much light is transmitted at a certain wavelength, emission is how much light is emitted at a certain wavelength.
Why are emission spectrophotometers generally more sensitive than absorbance techniques?
There is less background interference in emission spectra. It is comparing a baseline to 0 (something to nothing) whereas absorbance spectra compare big to something about as big. Emission changes are therefore more noticeable.
Discuss the major difference between how absorbance techniques and fluorescence (a type of emission) techniques depend on source intensity. How does each technique depend on the sample concentration and path length?
Absorbance is independent of light intensity as long as it is constant. Fluorescence, on the other hand, is directly proportional to light intensity.
Absorbance is directly proportional to concentration (think Beer’s law). Fluorescence is linear at low [ ]s, but decreases as too high [ ]s.
Absorbance is directly proportional to pathlength (think Beer’s law). Fluorescence is somewhat dependent on pathlength, but it’s not nearly as critical.
What are the disadvantages of fluorescence techniques?
Self-quenching at higher concentrations, results in lower signal.
Short lifetime.
Requires a very intense source
An advantage is much lower detection limits than absorbance techniques.
Know the advantages and disadvantages of absorbance and fluorescence (emission) techniques.
Absorbance
(+) Simple, wide range of applications, follows Beer’s law in proportionality to concentration
(-) Less sensitive at low concentrations, more prone to interferences, not ideal for complex samples, limited by Beer’s law and becomes non-linear at high concentrations
Fluorescence
(+) High sensitivity at low concentrations, can be linear of a broader concentration range, minimal sample prep, good for complex samples, reduced interferences, better selectivity
(-) Self quenching and decreased signal at higher concentrations, not all compounds fluoresce, more prone to background noise, expensive, shorter interference.
Know the advantages of a double-beam spectrophotometer over a single beam instrument.
Real time comparison with the reference blank compensates for fluctuation in light intensity/environmental factors, faster measurements, automatically corrects for any baseline drift.
Know the basic instrument set box diagrams for single and double beam spectrophotometers as well as for emission spectrophotometers. Draw it out.
Refer to notes for correct diagrams.
What is the main advantage of fluorescence techniques over absorbance? What is a disadvantage?
(+) Increased sensitivity and decreased detection limits.
(-) Sensitivity to interference (none to some).
What are some common sources used in spectrophotometry and what are the characteristics of each?
Tungsten-halogen lamp: smooth, continuous output with a long life and stable output (Visible and NIR absorbance).
Deuterium lamp: continuous UV spectrum with a stable output but has a relatively shorter lifetime (UV absorbance).
Laser: very intense, monochromatic, and coherent; great for excitation sources (Emission, fluorescence, IR).
Hollow-cathode lamp: used in atomic absorption, narrow lines (AAS for metals).
What are two different types of monochromators and which one is most commonly used today?
Prism: disperses light based on wavelength. (+) UV/Vis, simple. (-) non-linear, material-dependent.
Grating: grating with many spaced lines, diffracts light. (+) linear, wide UV → IR range. (-) overlapping peaks in complex alignments.
Grating most commonly used today.
Define and differentiate between the terms “dispersion” and “resolution” for grating monochromators.
“Dispersion” refers to how much the monochromator spreads out different wavelengths of light spatially.
“Resolution” refers to the monochromator’s ability to distinguish between two closely-spaced wavelengths.
Discuss how groove spacing on a grating relates to dispersion and resolution.
More grooves/mm (increased groove density, smaller groove spacing) = greater dispersion = higher resolution.
What are some typical sources used in IR spectrophotometric techniques?
Globar (Silicon Carbide Rod)
Nernst Glower
Incandescent Wire (Nichrome/Kanthal)
Tungsten Filament Lamp
Mercury Arc Lamp
Discuss the types of detectors used in spectrophotometric techniques and give the specific advantages.
Photodiode: UV-Vis; light generates a current across pn silicon junctions.
(+) simple, robust, single wavelength, cheap, long lifespan.
Photodiode arrray: UV-Vis; array of photodiodes = simultaneous detection of multiple wavelengths.
(+) fast, full spectrum at once, good for time-tight studies
Photomultiplier tube: UV-Vis, Fluoro; incoming photons hit cathode, multiplcation via increasingly positive dynodes.
(+) extremely sensitive, detects low light, fast.
(-) fragile, sensitive to ambient light.
Thermocouple: IR; converts heat from IR into voltage.
(+) simple, wide temperature range.
(-) less sensitive.
Pyroelectric: FTIR; crystal that generates current when heated by IR.
(+) fast, sensitive, good for modern FTIR.
Thermopile: IR; array of thermocouples, temperature rises.
(+) durable and cheap, more sensitive than a single thermocouple.
Discuss how slit width in a spectrophotometric instrument relates to sensitivity, noise, and resolution.
Slit controls amount of light entering the monochromator and exiting to reach the detector.
Inc. slit width = lower resolution because wavelengths aren’t spaced out, overlapping peaks = less spectral diffraction.
Inc slit width = more signal intensity because more light coming through.
Inc slit width = increased S/N because maybe more noise, but also more signal to offset that (S/N increase up to a point).
What does the acronym LASER stand for? Discuss how a LASER works? What are its advantages and disadvantages?
LASER = light amplification by stimulated emission of radiation.
Produces intense, monochromatic, coherent beam of light.
(1) Energy pumping/excitation = population inversion.
(2) Spontaneous emission, return to a ground state.
(3) Returning electrons cause stimulated emission of other electrons → amplification of light.
(4) Optical cavity, mirrors reflect light to amplify signal.
(+) High intensity so decreased concentration needed and signal detection limit, narrow bandwidth, high precision, minimal spread, coherence allow for interference techniques.
(-) Expensive, can damage samples, requires really specific alignment and safety measures.
What are the various materials from which a sample cuvette can be made? What are the important characteristics of each?
Plastic: Visible; (+) cheap and disposable, (-) scratches easy.
Glass: Visible and NIR; (+) durable and relatively cheap, (-) not UV transparent.
Quartz/fused silica: UV-Vis and some IR; (+) UV transparent and precise, (-) expensive and fragile.
IR-grade Quartz or CaF2: IR; (-) very expensive and fragile.
Sapphire: (+) harsh conditions, extremely durable, high temperature and chemical-resistant, (-) incredibly expensive.
What is Michelson interferometer and what is its only moving part? What is it used for?
Optical devise that splits a beam of light, reflects it along 2 paths, and then recombines it to make an interferences patter; only moving part is its MIRROR to vary the pathlength between two beams.
Used in FTIR spec to allow for fast and sensitive detection across a broad IR range, provides high resolution and high S/N ration, good sensitivity.
What is a Fourier Transformation? What types of instruments is it used in? What is the big advantage of using an FT instrument? What major discovery a few decades back made the use of FT instruments more feasible?
Computers and subsequent calculations made FT much more feasible.
Converts a complex signal into its component frequencies (turns an interferogram which is signal vs. mirror position or time into a spectrum of intensity vs. wavelength).
FTIR(IR), FT(NMR), FT mass spec.
(+) All wavelengths are measured simultaneously, so very fast; sensitive; no slits needed, so more light reaching the detector = better S/N; uses a laser as an internal reference so more precise.
What is signal averaging and how does it relate to the previous question?
Takes multiple scans of the sample sample, averages them to improve S/N.
How FT improves its S/N and resolution.
What is the signal-to-noise ratio in a spectroscopic signal and how can it be improved?
S/N = signal amplitude / noise amplitude.
Improved with signal averaging, increasing integration time, regularly calibrating, optimizing detector and source, and using narrower slits (to a point).
Why do atomic spectroscopic techniques show very narrow linewidths when compared with molecular techniques?
Atoms have simpler energy structures and only have electronic and not vibrational or rotational transitions which are usually what account for the broad peaks in molecular techniques.
Explain the “linewidth problem” encountered in atomic absorption when conventional sources/monochromators are used?
Absorption lines of atoms are super narrow, but the light from a conventional source or monochromator has a much broader linewidth. Only a small fraction of source light ends up actually overlapping with the absorption line, which can lead to poor sensitivity and a low S/N. To fix this, narrow linewidth sources like HCL are used so that they can emit sharp lines.
List the advantages and disadvantages of using the following in atomic spectroscopy techniques for the introduction of the sample: conventional flame, ICp, graphite furnace.
Conventional flame (AA, mostly):
(+) simple and cheap, rapid sample introductions, good for metal analytes.
(-) limited sensitivity, not a high enough temp for refractive elements, not good for elements with high excitation energies, high sample consumption.
ICP (AE, mostly):
(+) very hot (6000-10,000K) so good for efficient atomization and excitation, very low detection limits, simultaneous multi-element analysis, wide range. of sample compatibility.
(-) $, argon gas is also $, can have high background emission in some spectral regions (none against some).
Graphite furnace:
(+) very high sensitivity, very small sample volume required, trace metal analysis, bio and wet samples.
(-) slower, susceptible to matrix interferences, needs very careful temp programming, $ but still < ICP.
What advantages are gained from employing a beam chopper in a flame AA instrument?
Distinguishes light absorbed by sample from background emission and noise.
Eliminates flame and background emission interferences.
Improves S/N.
Allows use of lock-in amplifiers.
Gives more accurate AA measurements.
Be able to draw and/or label parts of an ICP ‘flame.’ What is the source of energy in this? Draw it out.
Refer to notes for correct diagram.
Energy source = ionized gas (usually Ar).
Very hot, 6000-10,000K.
Be able to draw the basic block diagram instrument setups for AA, AE, and AF spectroscopy.
Refer to notes for correct diagrams.
What is a lock-in amplifier?
A lock-in amplifier is a type of amplifier that extracts a weak signal buried in noise by comparing it to a known reference signal at the same frequency
It's highly sensitive and great for extracting signals in noisy environments, offering excellent signal-to-noise performance.
However, it only works well with periodic signals at known frequencies, can be complex to use, and may respond slowly due to averaging.
You add a lot of compound A to a 100mL flask, and dilute it to the mark. Because the solution is so concentrated, you take 2.00mL of this solution, place it in a 25.0mL flask, and dilute it to the mark. Some of this solution was placed in a cuvette and A was measured @ 238nm. The absorbance measured was 0.733, and the absorbance of the reagent blank was 0.029. What was the concentration in both the diluted solution and the original solution. The cuvette had a path length of 1.000cm and a molar absorptivity = 1502.5 M-1cm-1.
Cdil = 4.7 × 10-4 M
Cor = 59 × 10-3 M
There was some amount of copper added to an unknown solution measured with AA spectroscopy. Absorbance was measured to be 0.215. Then, a 25.00 mL aliquot of the solution was pipetted to a 50.0mL beaker and 5.000mL of 100.0ppm Cu2+ standard was added and mixed. This absorbance was measured to be 0.358. What was the concentration of the solution in the original equation?
20.0ppm
If you double the frequency of electromagnetic radiation, you do what to the energy?
Double the energy.
If you double the wavelength of electromagnetic radiation, you do what to the energy?
Halve it.
If you double the wavenumber of electromagnetic radiation, you do what to the energy?
Double it.
Which molecular processes correspond to the energies of microwave, infrared, visible, and UV EMR?
Microwave = rotation
Infrared = vibration
Visible = electronic transitions
UV = bond dissociation
Would you use a tungsten or deuterium lamp at 330nm radiation? What kind of light provides radiation with a 4 micrometer wavelength?
Deuterium, because 330 nm falls within the UV range and a tungsten lamp works mainly for Vis/IR at a hgigher wavelength range.
A 4 micrometer = 4000nm lamp falls within the IR range, so a tungsten-halogen or silicon carbide lamp would be the best.
Which variables increase the resolving power of grating? Which variables increase its dispersion? How is the blaze angle chosen to optimize a grating for a particular wavelength?
R = mN.
To increase resolving power, increase diffraction order and # of grooves. To increase dispersion, decrease groove spacing, increase diffraction order, and increase the diffraction angle.
What is the role of a filter in a grating monochromator?
A filter narrows down and selects a specific portion of the spectrum that the monochromator generates (makes it more precise).
What are the advantages and disadvantages of decreasing monochromator slit width?
(+) increased resolution because smaller wavelength range; sharper peaks; higher sensitivity to specific wavelengths because it’s working in a smaller range
(-) reduced intensity and may lower intensity of the detected signal; makes it harder to measure weak signals; longer measuring time because less light passing through; decreased intensity may increase relative noise; increased potential for stray light interferences.
What are the processes involved in atomic absorption of a solution of a metal ion? In atomic emission?
Absorption: gas is nebulized and put through a flame to atomize the sample; a hollow cathode lamp emits a wavelength that the metal ion can absorb; detector measures intensity of light before and after it passes through the flame.
Emission: sample is nebulized, a high-temp excitation source like a plasma or laser is used; atoms absorb thermal energy from the plasma; excited state; excited state atoms are unstable so they emit photons; emitted light separated by the monochromator.
State the advantages and disadvantages of a furnace compared wit a flame in atomic absorption spectrometry.
(+) increased sensitivity due to longer residence time in the optical path, better trace analysis.
(-) slower, more complex, $$, more prone to matrix interferences and may require chemical modifiers or background correction.
State the advantages and disadvantages of inductively-coupled plasma with a flame in atomic absorption spectrometry.
(+) much higher sensitivity and precision; can analyze multiple elements at once; fewer interferences with high and stable temperatures; suitable for complex samples.
(-) $$, overkill for single element or simple samples.
WAVELENGTH AND ENERGIES PLS!
Know wavelengths from table, can get energies from wavelengths.

Describe MP/LP of partitioning chromatography.
SP = Liquid; MP = Liquid/gas.
Describe MP/LP of adsorption chromatography.
SP = Solid; MP = Liquid/gas.
Describe MP/LP of ion-exchange chromatography.
SP = Bonded ions; MP = Liquid.
Describe MP/LP of molecular exclusion techniques.
SP = Porous (ex., gel); MP = Liquid.
Describe MP/LP of affinity separation.
SP = Antibody/antigen; MP = Liquid.
What is the purpose of a solvent-solvent extraction and what are the two layers?
Process to transfer dissolved solutes from one phase to another. Involves (1) aqueous/polar layer and (1) organic/nonpolar layer, each of which will provide solutes of interest with different levels of solubility. Solvents must be immiscible.
Define the partition coefficient and its equation.
Partition coefficient, K, measures relatively solubility of a solute in the two different phases.
K = [S]org / [S]aq
Define “q” and explain how it relates to [S]org and [S]aq.
“q” is the fraction of solute remaining in the aqueous phase after 1 extraction.
[S]aq = q x (m/V1) = mol/L = M.
[S]aq = (1 - q) x (m/V2) = mol/L = M.
Are “q” and “K” related to one another?
Yes, they are. Equation on equation sheet.
How can we measure the fraction of solvent remaining the aqueous after some “n” number of extractions? How does increasing the number of extractions impact extraction efficiency?
qn = q n = (V1 / KV2 + V1)n [the second part of that will be on the equation sheet].
Doing multiple small extractions is more efficient (results in smaller q) than doing one or just a few big extractions.
Describe the pH effects when performing solvent extractions with organic acids and bases? How does this related to the distribution constant, D?
The aqueous phase is polar and will promote the dissociation of acids/bases into their ionic forms (ex., HA will want to turn Into H+ and A- in the aqueous phase).
The nonpolar organic phase does not like ionic forms.
D is kind of like K, but for organic acids/bases only. In the equation book, but
D = K / 1 + (Ka / [H+])
This tells us that the distribution coefficient for acids/bases is really a function of their pH.
How do D and K related to each other in extreme conditions?
If [H+] is MUCH greater than the Ka, then the pH is MUCH less than the pKa. So, approx…
D = K
If [H+] is MUCH less than the Ka, then the pH is MUCH greater than the pKa. So, approx…
D = K[H+]/Ka and it approaches = 0
Be able to interpret and understand distribution curves for organic acids/bases.
D on y-axis, pH on x-axis.
Organic acids…
Starts high, at K (partition coefficient)
Asymptote @ pKa.
Before pKa, acid still protonated (HA), neutral, prefers organic phase.
After pKa, deprotonated (A-), polar, now prefers aqueous phase.
Organic bases…
Starts low, at the K.
Asymptote @ pKa.
Before pKa, base protonated (BH+) and polar, prefers organic phase.
After pKa, base now deprotonated (B), neutral, prefers organic phase.
Why do we salt out extractions?
Many organic maybe be largely nonpolar but still have some solubility in the aqueous phase. Add NaCl (or any other salt) to the aqueous layer, basically filling it so much with salt ions that the phase won’t want the solute that much anymore.
Pushes or “salts out” the compound to the organic layer so increased [S]org, improves separation, increases K.
What type of chromatography uses an open tubular column? What does the even mean?
Used in partition chromatography.
Open hollow tube, has solute dissolved in a liquid phase that binds to the column’s interior surface.
Define the difference between eluent and eluate.
Eluent goes in, eluate comes out.
What are the MP/SP for partition and adsorption chromatography in liquid chromatography?
Partition (OT): Liquid/Liquid.
Adsorption: Liquid/Solid.
What are the MP/SP for partition and adsorption chromatography in gas chromatography?
Partition (OT): Gas/Liquid.
Adsorption: Gas/Solid.
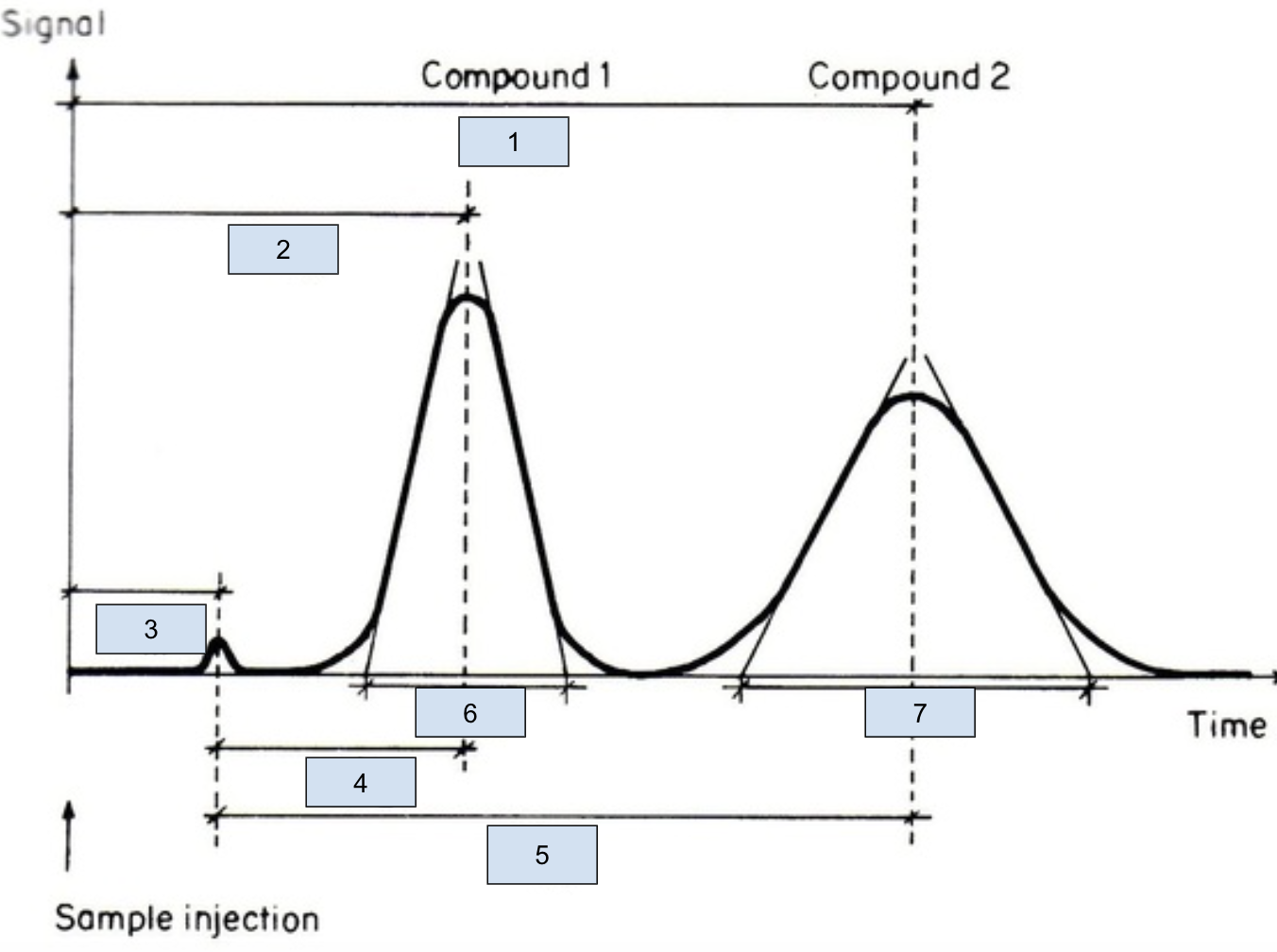
Be able to label all components of this chromatogram. Will need to identify times and widths for calculations.
tr1
tr2
tm
t’r1
t’r2
w1
w2
What is the retention volume?
Retention volume, Vr, is the volume of MP needed to elute a particular solute.
What is the volume flow rate (mL/min)?
Volume flow rate, uv = F, is how many mL of solvent flow through the column each minute.
What is the linear flow rate (cm/min)?
Linear flow rate, ux = v, is how many cm of the column length a solvent travels each minute.
What is the difference between retention factor, k, and partition coefficient, K?
k = K(Vs/Vm), K = [S]2/[S]1
“k” describes the time spent in two phases (mainly, also # moles).
K compares the concentration of solutes in phases.
For a particular solute, k = (tr - tm)/tm
a higher k means the solute was retained longer.
k = 0 means solute wasn’t retained.
Do all solutes spend the same time in the mobile or stationary phase?
Same time in the MOBILE phase. Time in stationary phase depends on chemical properties of solute compared to SP material.
What is relative retention? What does it tell you.
α = t’r2 / t’r1 = k2 / k1
Compares adjusted retention times of two solutes.
The greater α is, the more the two compounds have been separated.
Calculation for retention volume?
Vr = tr x F (min x mL/min = mL)
What is peak resolution?
Peak resolution is the ability to distinguish between two different, adjacent peaks on a chromatogram. Can be calculated as:
R = Δtr / wavg
= ΔVr / wavg
= 0.589Δtr / w1/2 av
The greater the R, the better the chromatogram is. Goal is to maximize Δtr and minimize widths. Baseline resolution is 1.50.
What is separation efficiency measured in?
Theoretical plates, N. The goal is to maximize the N.
N = 5.55tr2 / w1/2 2
w1/2 = 2.35σ
= 16tr2 / w2
w = 4σ
= tr2 / σ 2
As peaks move down the column, there are many factors that cause them to spread out. What is this called and how does this affect separation efficiency?
Band broadening.
Worsens separation effeciency by (a) lowering the N and (b) reducing R for closely placed peaks.
What is theoretical plate height? How does this related to N and separation efficiency?
H = L / N (unit of height).
As plate height decreases, resolution increases. Want to minimize the plate height (because that means there are more plates and better separation).
What is the van Deemter equation?
H = A + B/ux + Cux
A = “multiple paths”
B = longitudinal diffusion
C = equilibration time
ux = flow rate
causes decrease in B-term
causes increase in C-term
need a balance of flow rate!
A, B, and C are all reasons of band broadening (greater plate height).
Describe what the A-term in van Deemter is.
*only applicable to packed columns, for an OT column A = 0.
A very packed column makes the MP take a twisty and therefore longer route → this causes band broadening.
Solution to minimize A for H: use smaller, uniform particles and density.
Describe what the B-term in van Deemter is.
How much the solutes naturally diffuse as they’re in the column.
Decreases as flow rate increases → faster means less time to diffuse.
Smaller molecules also diffuse more/faster.
Describe what the C-term in van Deemter is.
It’s the time needed to equilibrate. C-term increases with flow rate.
List methods to increase N (minimize H).
Optimal flow rate (not necessarily largest).
Smaller SP particles for non-OT columns.
Use OT over packed when possible to just eliminate A-term altogether.
Thinner SP in OT or packed → makes equilibrium easier, lowers C-term.
Longer column (N = L/H).
What is overloading/fronting caused by and what does it look like?
Overloading/fronting is when [solute] too hight, so SP and solute kind of start looking the same. (peak leans forward on chromatogram).
What is tailing caused by and what does it look like?
Tailing is caused by unwanted solute-SP interactions. Solute held back by SP (peak leans back).
What is required of a solute to be able to perform GC on it?
Solute must be volatile.