Open systems (first law of thermodynamics)
1/25
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
26 Terms
For a steady flow process, what is true about the mass?
the total amount of mass contained within a control volume doesn’t change with time
what does the conservation of mass require?
the total amount of mass entering a control volume = the total amount of mass leaving
what are the equations for mass flow rate in steady flow processes?
mass flow rate in = mass flow rate out ( = p1 x V1 x A1 = p2 x V2 x A2)
for an incompressible liquid, what is the mass balance?
Volume flow rate in = Volume flow rate out (=V1 x A1 = V2 x A2)
For the steady flow of liquids what is true?
volume flow rates and mass flow rates stay the same since liquids are essentially incompressible substances
what is flow work/flow energy?
the work required to push the mass into or out of the control volume. This work is necessary for maintaining a continuous flow through a control volume
what is the equation for work flow
Work (flow) = FL = PAL = PV
what is the sum of energy of a non-flowing fluid?
energy = u + ke + pe = u + V²/2 + gz
what is the sum in energy if a flowing fluid?
θ = PV + e = PV + (u +ke +pe)
what is the sum of energy including enthalpy
θ = h + ke + pe = h + V²/2 + gz
what is a steady flow process?
a process during which a fluid flows through a control volume steadily
show the energy balance for a steady-flow process
rate of energy in = rate of energy out
rate of heat (in) + rate of work (in) + rate of mass x energy (in) = [—-]out
what is the energy balance for a non-steady flow process?
rate of heat (in) + rate of work (in) + rate of mass x energy (in) = [—-]out + ΔE
what is a nozzle? draw it!
a nozzle increases the velocity of a fluid at the expose of pressure
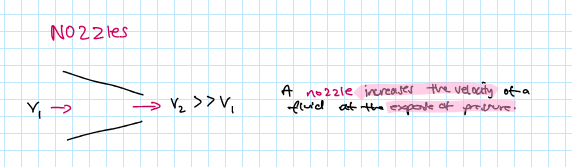
what is a diffuser? draw it!
a diffuser increases the pressure of a fluid by slowing it down
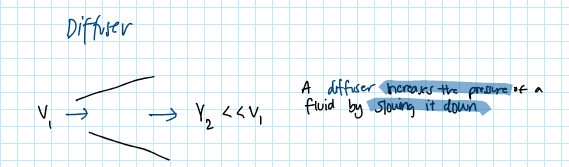
How does the CSA of a nozzle work to produce it’s function?
the CSA decreases in the flow direction for subsonic flows and increases for supersonic flows vice versa for diffusers
what is the energy balance for a nozzle/diffuser?
rate of energy in = rate of energy out
rate of mass flow x (h1 x V1²/2) = mass flow rate x (h2 x V2²/2)
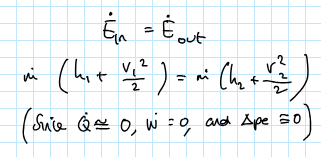
what is a turbine?
drives the electric generator in steam, gas or hydroelectric power plants. as fluids passes through a turbine, work is done against the blades attached to the shaft, thus, the shaft rotates and the turbine produces work.
what is a compressor, pump or a fan?
increases the pressure of a fluid
work is supplied to these devices from an external source through a rotating shaft
what is the specific function of a fan?
increases the pressure of a gas slightly and is mainly used to mobilise a gas
what is the specific function of a compressor?
compresses the gas to higher pressures
what is the specific function of a pump?
compresses liquids to higher pressures
What is a throttling valve?
any flow-restricting device that causes a significant pressure drop in the fluid
In refrigeration and AC applications, why is a throttling valve used?
the pressure drop in the fluid is usually followed by a large drop in temperature
what is the energy balance for a throttling valve?
u1 + p1V1 = u2 + p2V2
for ideal gases in a throttling valve, what is true regarding temperature?
the temperature of the ideal gas doesn’t change ( h = constant)