12 - Countercurrent Mechanism of Distal Tubule and Collecting Duct
1/12
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
13 Terms
countercurrent mechanism
mechanism for kidney concentrating urine
kidney can concentrate urine 4-fold (1200 mOsm/L) or dilute urine 7-fold (40mOsm/L) with respect to plasma
plasma in osmotic equilibrium at 290 mOsm/L
countercurrent components
glomerulus → filtrate is iso-osmolar (290 mOsm/L)
proximal tubule → 2/3 reabsorption of water and solutes
descending limb → filtrate becomes hyper-osmolar (1200 mOsm/L)
water reabsorption via aquaporin 1 and paracellular transport
impermeable to solutes
ascending limb
NaCl reabsorbed paracellularly
impermeable to water
thick ascending limb → filtrate becomes hypo-osmolar (100mOsm/L)
solute reabsorption of Na+, K+, and Cl- via Na+-K+-2Cl- transporter
impermeable to water
distal tubule and collecting duct → solute and water reabsorption is tightly regulated
osmolality of final urine is a function of the degree to which the fluid in collecting ducts is allowed to equilibrate with interstitium
hyperosmotic gradient
as filtrate moves from cortex to medulla, filtrate becomes more concentrated
50% of gradient due to urea
urea recycling (reabsorbed and secreted) between collecting ducts and loop enhances gradient
50% of gradient due to NaCl
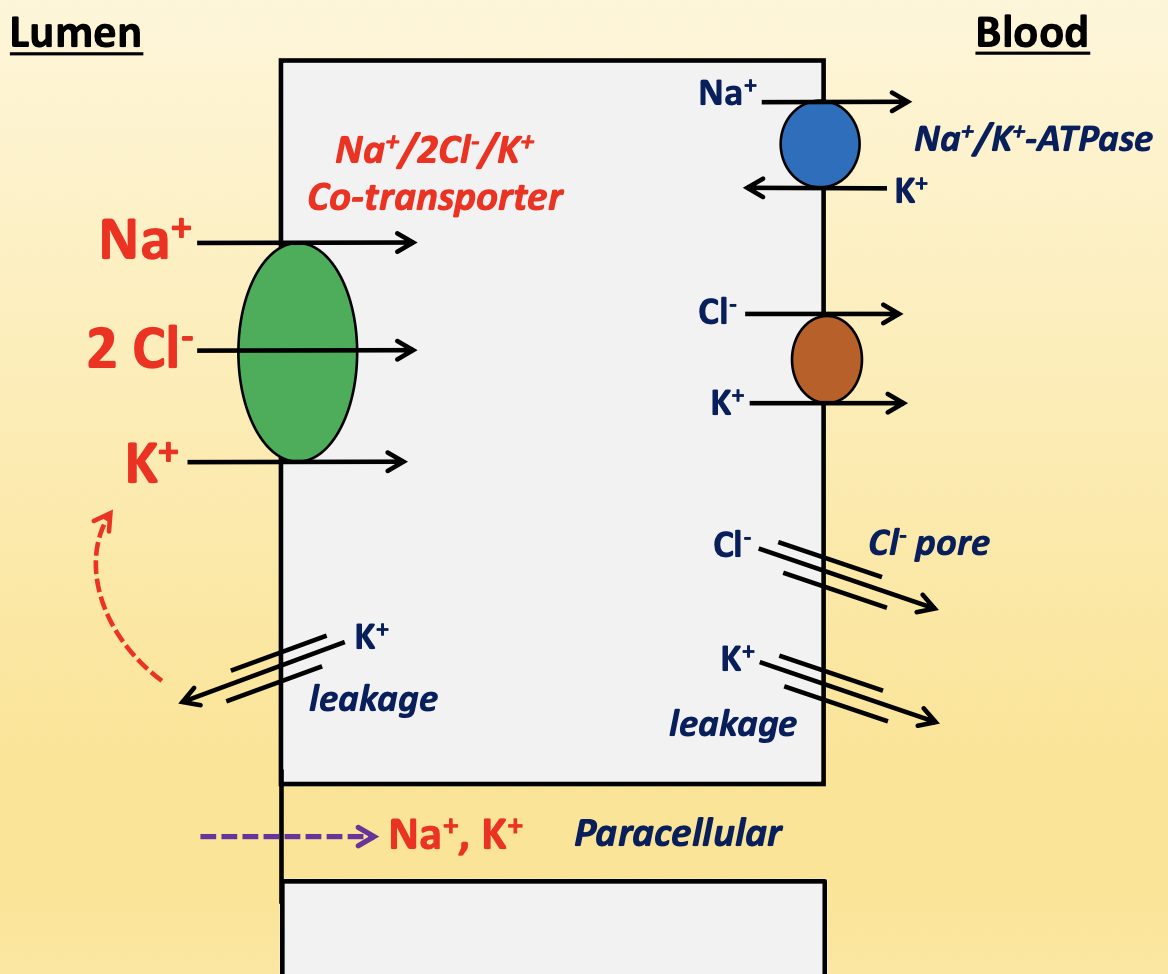
thick ascending limb
cells do not have brush border, so reabsorption is not as abundant
Na+/2Cl-/K+ co-transporter (apical)→ macula densa sensor that detects NaCl in filtrate for TGF response
responsible for 20-30% of hyperosmotic gradient
K+ back leak is key to keep the transporter working, but also creates a negative charge in lumen to push Na+ and K+ paracellularly
ADH increases activity of transporter
diuretic: furosemid (Lasix)
Na+/K+-ATPase (basolateral) → pumps Na+ out and K+ in
Cl-/K+ co-transporter (basolateral) → pumps Cl- and K+ out
K+ and Cl- pores (basolateral) → leaks K+ and Cl-
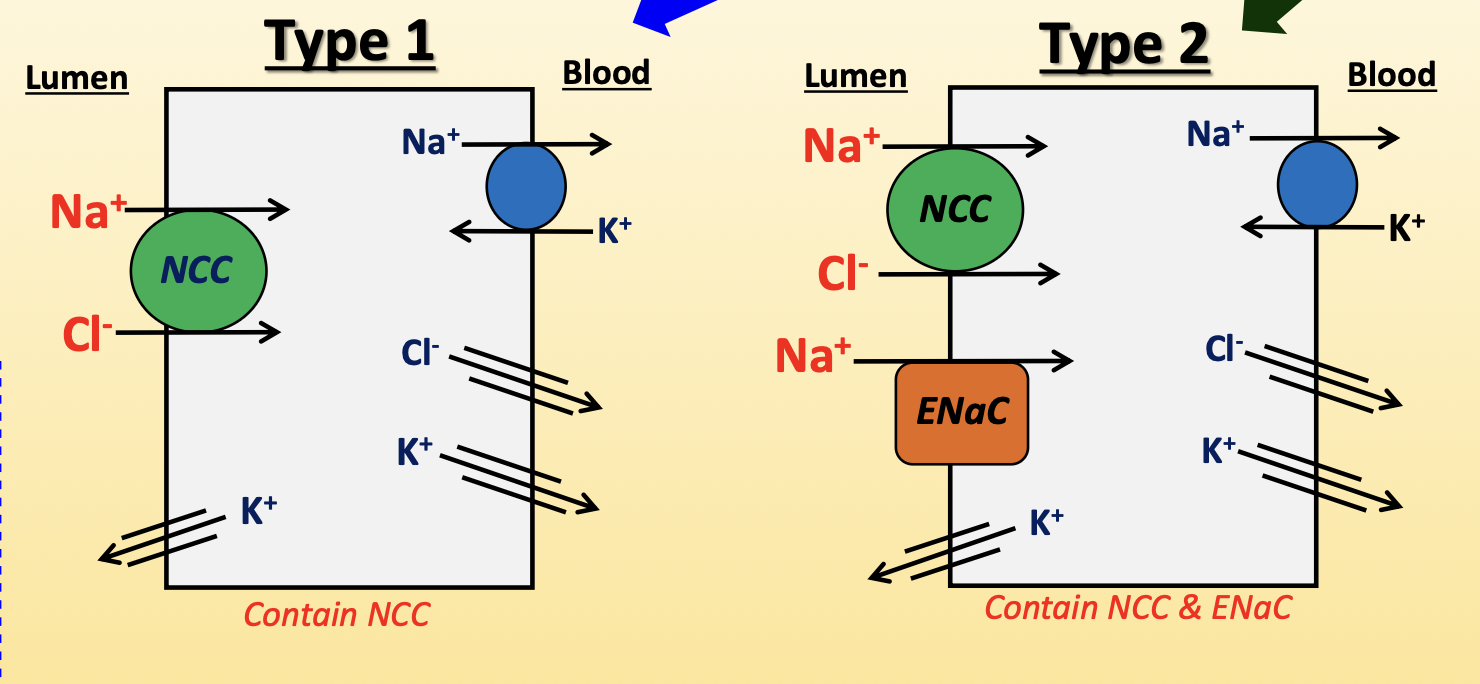
convoluted distal tubule
contain type 1 cells closer to macula densa, and type 2 cells closer to collecting duct
type 1 cells:
Na+/Cl- co-transporter (NCC, apical) → pumps Na+ and Cl- into cell
upregulated by AngII, aldosterone, ADH
diuretic: thiazide derivatives
Na+/K+-ATPase (basolateral) → pumps Na+ out and K+ in
Cl- pores (basolateral) → leaks Cl- into blood
K+ leaky channels (apical/basolateral) → leaks K+ into blood and lumen
type 2 cells:
NCC (apical)
epithelial Na+ channel (ENaC, apical) → pumps Na+ into cell
upregulated by aldosterone and increased tubular flow
downregulated by ANP
diuretic: spironolactone and amiloride
Na+/K+-ATPase (basolateral) → pumps Na+ out and K+ in
Cl- pores (basolateral) → leaks Cl- into blood
K+ leaky channels (apical/basolateral) → leaks K+ into blood and lumen
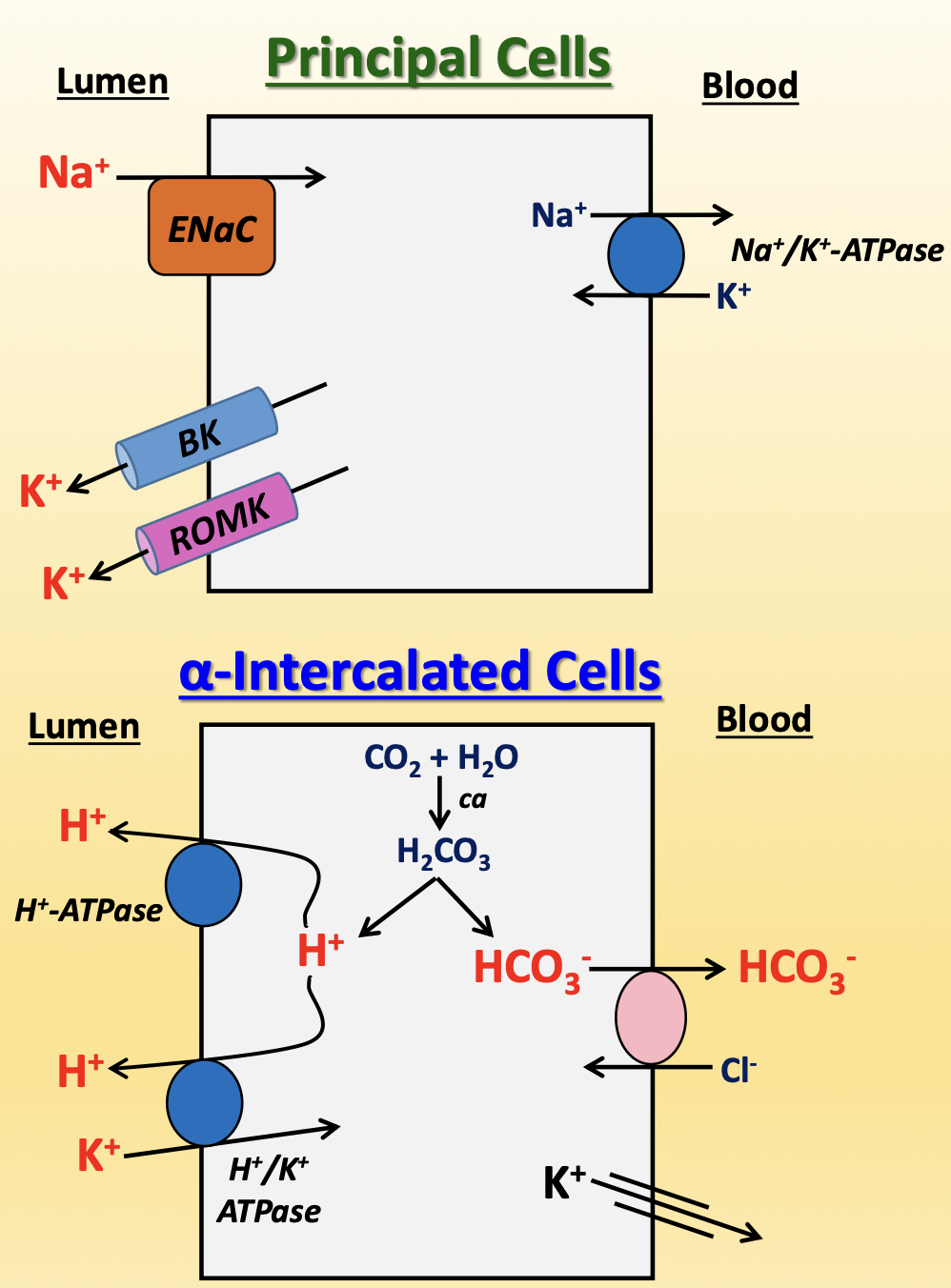
collecting duct
contain principal cells and intercalated cells, with more principal cells at 2:1 to 3:1 ratio
principal cells
ENaC (apical)
K+ excretion (apical) → Big K+ (BK) channel and renal outer medulla K+ (ROMK) channels
aquaporins (apical/basolateral)
Na+/K+-ATPase (basolateral)
⍺-intercalated cells (99% of intercalated cells)
reabsorbs HCO3-
excretes H+ (apical) → H+-ATPase and H+/K+-ATPase (counter-transporter)
reabsorbs K+ (basolateral) → leaky channels to compensate for high K+ excretion from principal cells
β-intercalated cells (1% of intercalated cells)
opposite polarization → secretes HCO3- and reabsorbs H+
diuretics
drugs that increase urine production by blocking solute reabsorption, increasing amount of sodium and water in filtrate to be excreted as urine
Na+/2Cl-/K+ → loop diuretics (furosemide/Lasix)
NCC → thiazide derivatives (chlorothiazide, hydrochlorothiazide)
ENaC → amiloride, spironolactone
osmotic → mannitol
carbonic anhydrase → blocks HCO3- reabsorption in proximal tubule
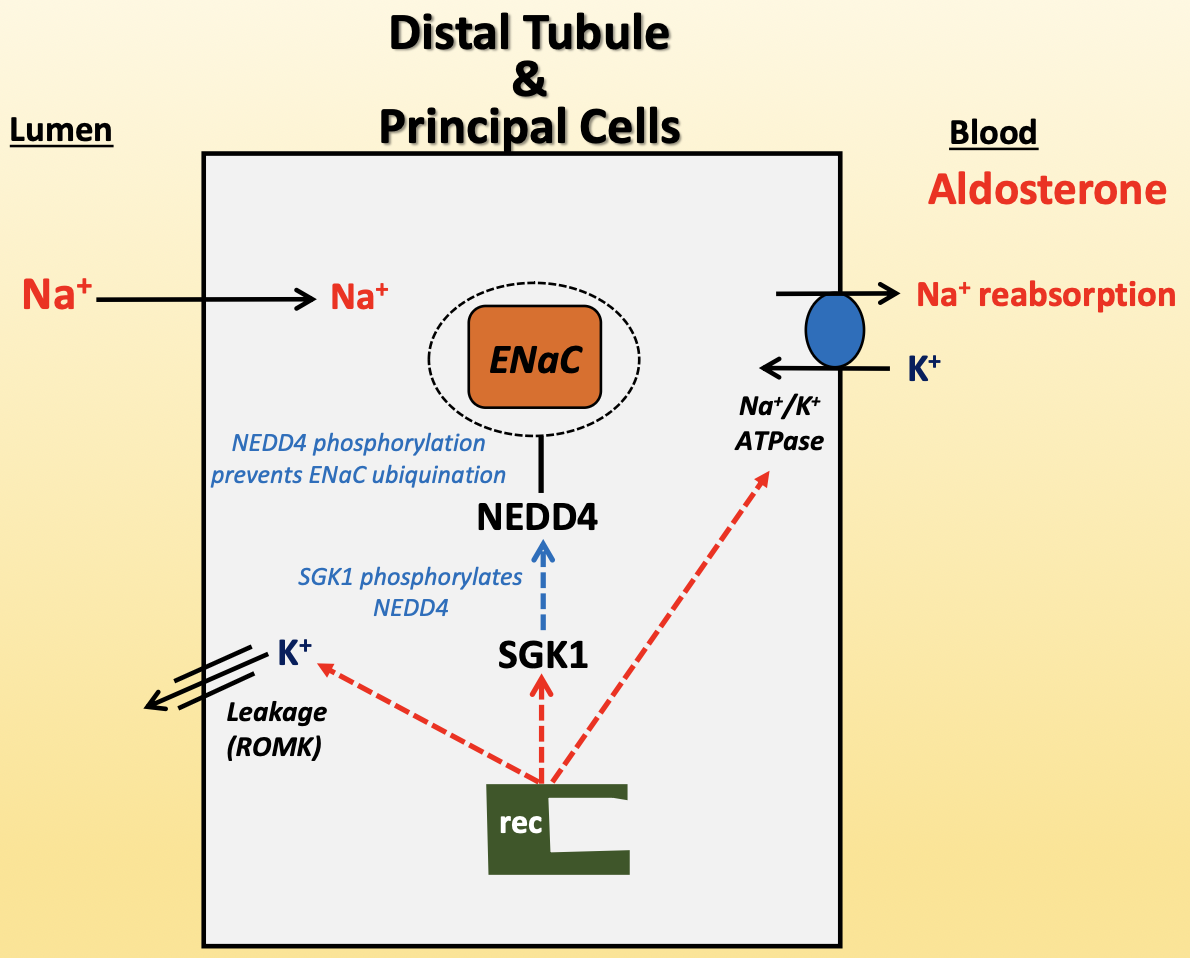
aldosterone
increases Na+ reabsorption and K+ secretion
aldosterone activates intracellular receptor
increases ROMK expression
increases Na+/K+-ATPase expression
SGK1 expression
active SGK1 phosphorylates NEDD4
NEDD4 phosphorylation prevents ENaC ubiquitination for degradation
released from adrenal gland in cortex
AngII activates its release during volume depletion and lowered BP
increased K+ activates release
aldosterone pathologies
hyperaldosteronism → Conn’s syndrome
33% caused by adrenal adenoma, 66-67% caused by enlarged hyperplasia of adrenal gland
increased Na+ reabsorption, leading to increased BP
hypoaldosteronism → Addison’s syndrome, diabetic nephropathy
how would increase in Na+ intake affect aldosterone levels?
aldosterone would decrease → to decrease Na+ reabsorption
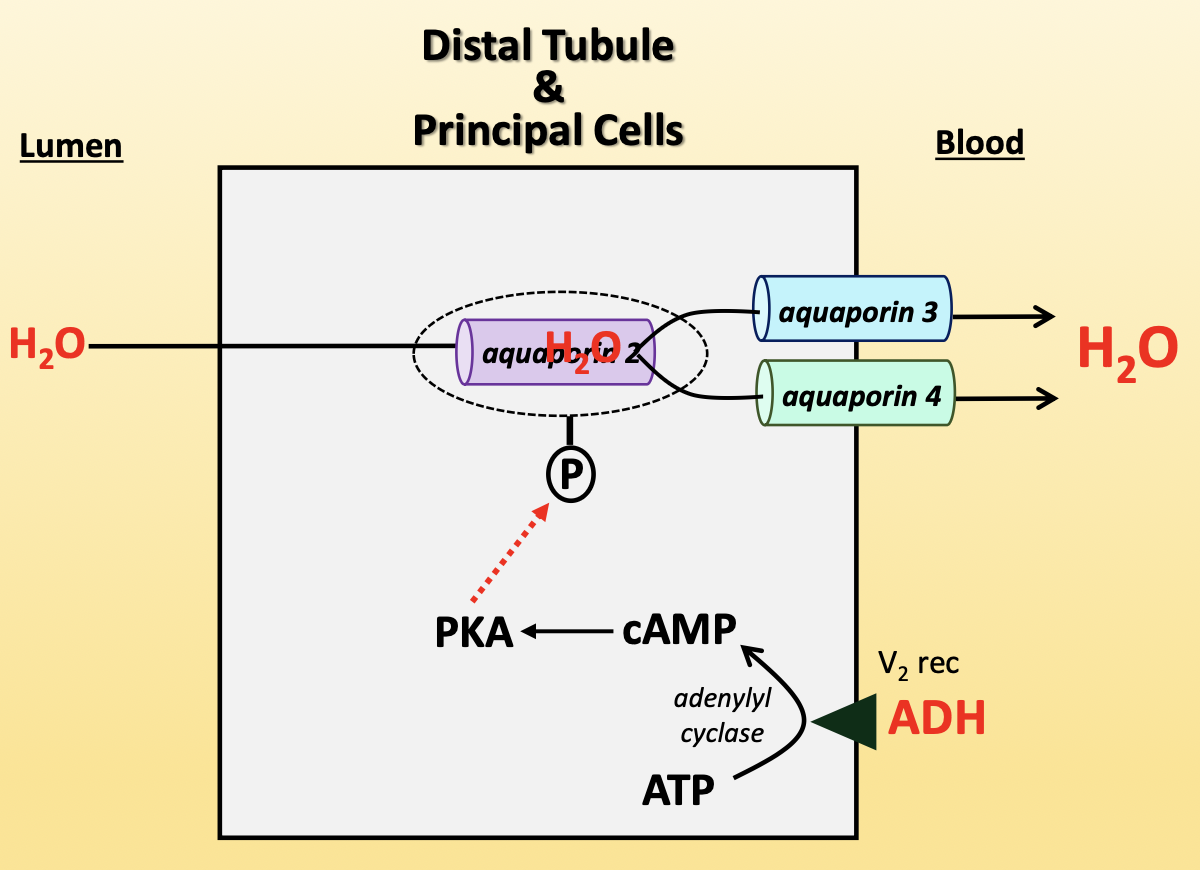
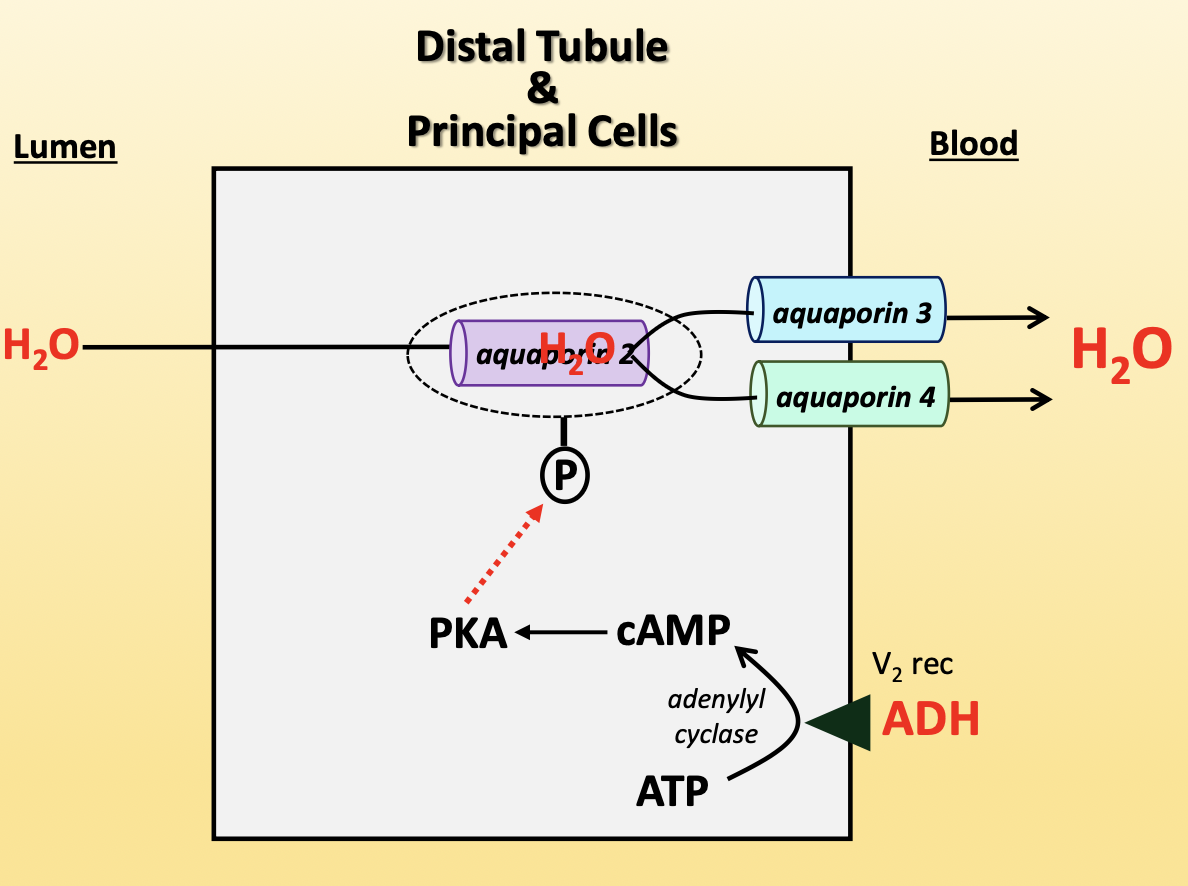
antidiuretic hormone (ADH)
increases water reabsorption in kidneys
ADH binds V2 receptor on basolateral membrane
activates adenyl cyclase to convert ATP to cAMP
cAMP activates PKA
PKA phosphorylates aquaporin-2 vesicle, allowing aquaporin-2 to insert into apical membrane
aquaporin-3/4 (basolateral) → constitutively present
increases activity of NCC, ENaC, Na+/K+-ATPase
released from posterior pituitary
sensed by baroreceptors during volume depletion
sensed by osmoreceptors when plasma osmolarity increases
ADH pathologies
central diabetes insipidus
ADH not around and not released from pituitary gland
from head injury
causes hypo-osmotic urine and increased tubular flow rate
nephrogenic diabetes insipidus
ADH released from pituitary but kidneys do not respond to it
from genetics, kidney disease, lithium
causes hypo-osmotic urine and increased tubular flow rate
ADH and extracellular fluid
normally → as plasma osmolality goes up, ADH goes up
body prioritizes correcting plasma volume over plasma osmolality
volume contraction → ADH released more significantly to retain volume
volume expansion → ADH release limited to correct osmolality
morphine and nicotine increase ADH release, alcohol decreases ADH release