JBC Stereochemistry
1/34
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
35 Terms
Define empirical formula
Simplest whole number ratio of elements present in a substance.
Define molecular formula
Actual number of each element in a molecule
Define constitution
Molecular connectivity of a molecule
Define configuration
Permanent spatial arrangement within a molecule
Define conformation
Spatial arrangement within a molecule caused by rotation/torsion
Define conformation
Spatial arrangement within a molecule caused by rotation/torsion.
Configuration isomers
Stereoisomers which can be interconverted by breaking and making bonds
Conformational isomers
Stereoisomers which can be interconverted by rotation about single bonds
What is the Newman Projection
Alternative to dash-wedge notation. Drawing the molecule as if looking down the c-c bond.
Define hyperconjugation
Repulsion between H atom electron clouds
Define heat of combustion
Change in thermal energy from conversion of alkanes to co2 and h2o. Can be measured by calorimetry.
Define heat of formation
Change in thermal energy from formation of 1 mole of a substance under standard conditions. Can be calculated from known enthalpy of combustion and enthalpy of formation.
Define Hess’ Law
Entropy changes are additive
Why are cyclopropane and cyclobutane unstable?
Due to ring strain
What does ring strain mainly come from?
Angle strain
Tortional strain
Structure of cyclopropane
Planar molecule
Angle strain - bond angles 60, so 49.5 degrees away from ideal (109.5 tetrahedral)
Torsional strain - 3 eclipsed c - c bonds so 2×2.8kcalmol-1
Total strain - 27.6 Kcalmol-1
Structure of cyclobutane
Angle strain - Bond angles approx 90 so 19.5 away from ideal
Tortional strain - 4 x nearly eclipsed c - c, 4 x 2.8kcalmol-1
Total strain = 26.3 kcalmol-1
Cyclohexane (chair)
In the chair confirmation, eclipsing of the hydrogens is completely prevented, the c-c-c bond angles are very nearly tetrahedral and the molecule is nearly strain free.
Cyclohexane (boat)
A second less stable conformation of the cyclohexane.
Less stable than the chair formation by 6.9kcalmol-1
Higher energy is due to the eclipsing of the 8 hydrogens at the base of the boat and the transannular (steric crowding across a ring) strain between the 2 H’s at the top.
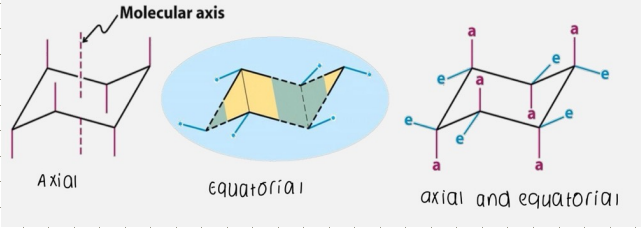
Axial and Equatorial positions on cyclohexane (chair)
In the chair form cyclohexane has 2 types of hydrogens:
Axial - 6 H parallel to the principle molecular axis
Equatorial - 6 H perpendicular to the principle molecular axis
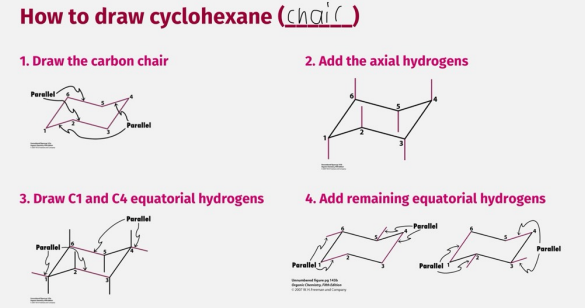
How to draw cylohexane (chair)
Flipping cyclohexane
The conformational flipping between 2 chair forms interconverts the equatorial and axial positions
If Ea low enough for rapid interconversion at room temp, substituents may favour one conformation, affecting stereochemistry and reactivity.
Monosubstituted cyclohexanes
Lowest energy chair formation - the substituent in an equatorial position
Chair flipping equilibrium shifts towards equatorial conformation
Equilibrium shift determined by size and nature of its substituent.
Chirality
Non super imposable on its mirror image
Enantiomers
Pair of non superimposable mirror images of a chiral molecule.
Same chemical properties
Stereocentres
A chiral molecule has at least one stereogenic centre
Organic chiral molecules usually have an atom that is connected to 4 different substitute group
This atom is called the asymmetric carbon or a stereocentre
How to identify chirality?
Symmetry helps to distinguish chiral structures from achiral ones
For most organic molecules a sufficient test for chirality is absence of a plane symmetry.
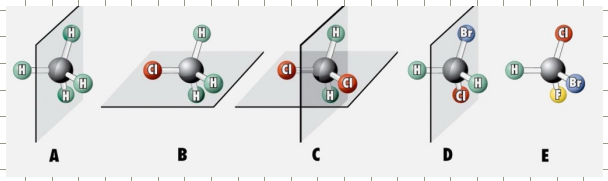
Experimentally identifying chirality
Enantiomers have the same physical properties (mp, bp, solubility etc) but they interact with plane polarised light differently
The rotation can be measured using a polarimeter to give the observed rotation.
The rotation is affected by path length (l) and concentration (c)
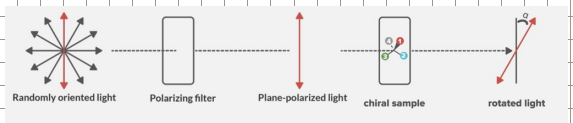
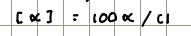
Dextrorotary enantiomers rotate light…
clockwiseA
Levorotary enantiomers rotate light…
counterclockwise
Define racemic/racemate
Equal amounts of both enantiomers
No net rotation in polarised light ([a]=0)
Equilibration of enantiomers is called racemisation
Diasteromers
Two or more stereocentres
When do diastereomers typically arise
When there is more than one asymmetric centre (stereocentre) in a molecule
E/Z isomers of double bonds are always diasteromers.
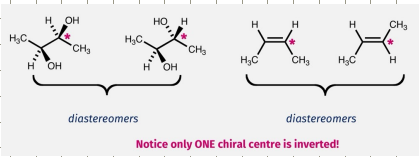
Enantiomer vs diastereomer
