BS132 - Lecture 2 - Cycloalkanes
1/7
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
8 Terms
What would happen if Cyclobutane followed Planar Conformation?
Angle
Compression and its effect
Torsional Strain
How is Planar Conformation different?
90 degree angle
Less compression of angle - better overlap so stronger bonds than cyclopropane
Max torsional strain - CH sigma bonds eclipsed
Planar conformation gives less angle strain but equal torsional strain
Puckered/Bent Conformation in Cyclobutane
Why doesn’t Cyclobutane adopt Planar Conformation?
What is Puckered Conformation?
Effect on Ring Strain
Effect on Torsional and Angle Strain
What is the result?
All H eclipsed
1 C out of plane
Relieves overall ring strain
Decreases torsional strain, increases angle strain
Relief of torsional strain > increase in angle strain compared to planar structure to give lower ring strain
Puckered Conformation in Cyclopentane
Envelope
Effects on angle and torsional strain
1 C out of plane, gives envelope conformation
Increases angle strain, relieves torsional strain
Ring Strain in Cyclohexane
How does it compare to others?
Angle Strain
Torsional Strain and what it means
Lowest ring strain - 0
No angle strain so internal angles have to be 109.5
Minimal torsional strain - CH bonds on neighbouring Cs are staggered, not eclipsed
Chair Conformation of Cyclohexane
Adpoted instead of?
Bond Angle
Effect on Angle Strain
Staggering
Torsional Strain
Ring Strain
Adopted instead of planar
C-C-C Bond Angle = 109.5
Leads to free angle strain
Perfect staggering of CH bonds on C-
Minimal torsional strain - 60degrees
No ring strain - No angle/torsional strain
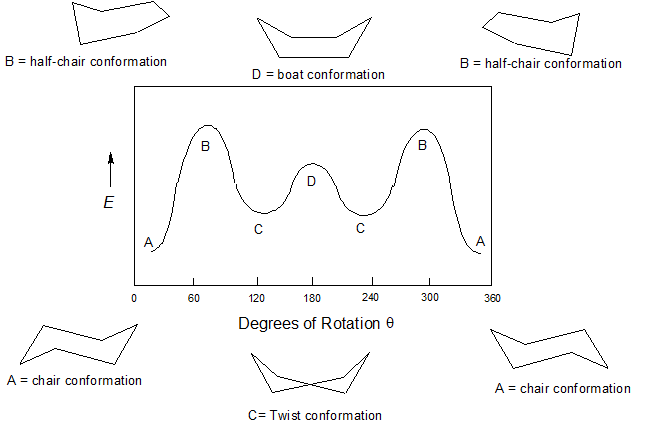
Boat Conformation
Angle strain?
Torsional strain
Transannular Strain
No angle strain, other types contribute to ring strain
Torsional strain due to eclipsing of 8 H atoms at base
Transannular (across ring steric) strain due to vdw repulsion between flagpole H atoms
Separation of Cyclohexane
Room Temperature
How significant is chair conformation
Separation of cyclohexane confromers impossible at room temp
99% of molecules in chair confirmation at any given moment
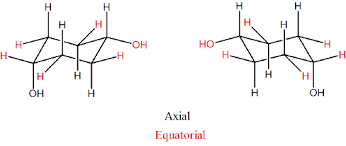
Substituted Cyclohexanes
Position of CH3 and its effect
What does Axial mean?
What does Equatorial mean?
How do they compare
Conformation w/ CH3 in axial position is less stable due to steric strain (vdw repulsion) experienced from H atoms at axial positions on C3 and C5
Axial - points up
Equatorial - Away from middle
Equatorial more stable chair - steric strain introduced between 2 Hs on C3 and C5 (known as 1,3-diaxial interactions)