p6
1/7
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
8 Terms
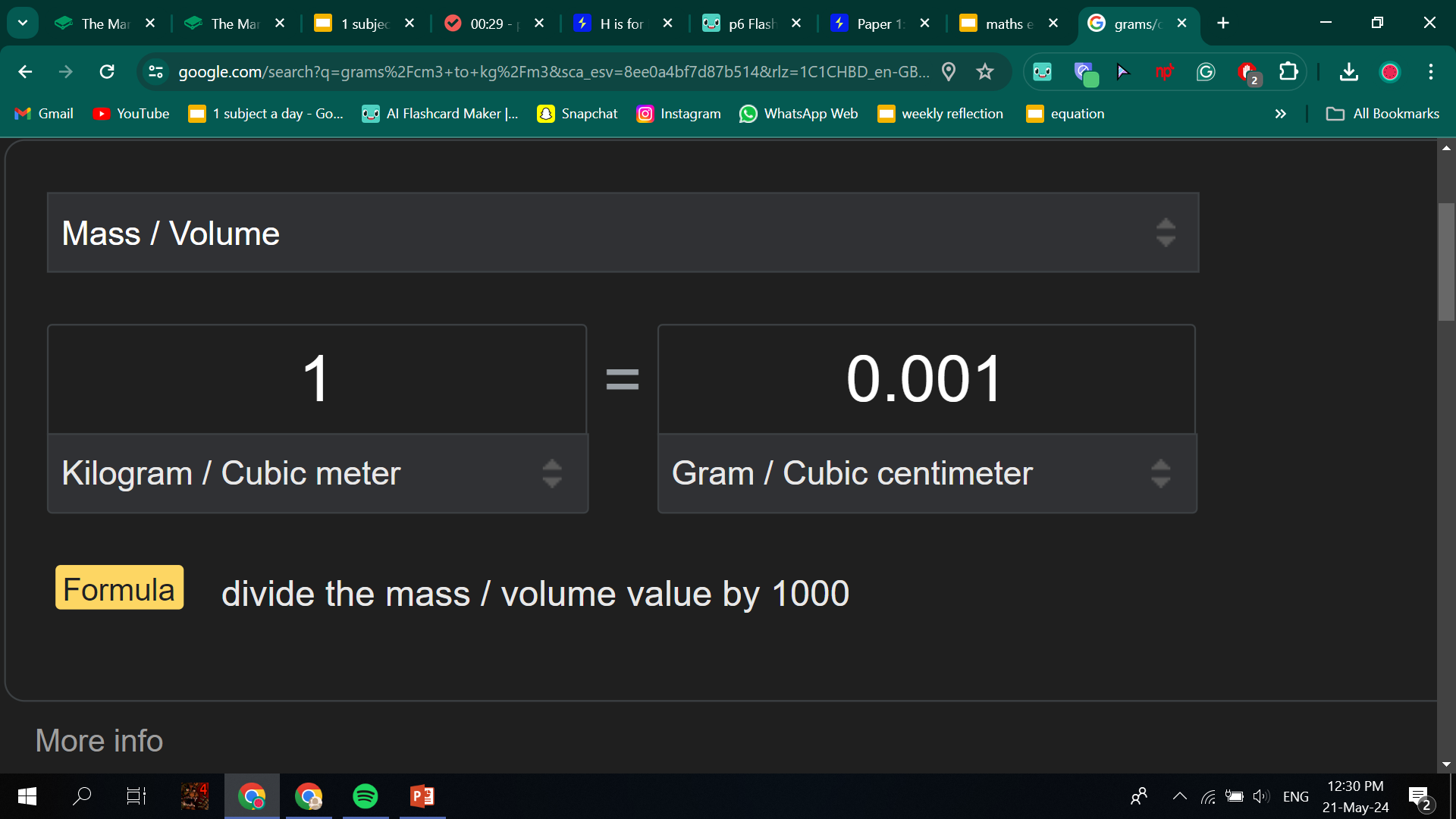
To convert g/cm3 to kg/m3 you have to:
To convert from g to kg you need to divide by 1,000.
To convert from per cm3 to per m3 you need to multiply by 1,000,000 (because a m3 is 1,000,000 larger, so there will be 1,000,000 x more particles in it).
So overall, we have have to multiply by 1,000
density of a solid
Measure the mass of the solid using a balance.
If the shape is regular, measure the volume of the solid using geometry.
If the shape is irregular, measure the volume of the solid by adding it to a Eureka can filled with water. This will cause a volume of water exactly equal to the volume of the solid to flow into the measuring cylinder.
Use the formula to calculate the density of the solid from the mass and volume measurements.
The specific latent heat
is the energy required to change 1kg of a particular substance from one state to another, without a change in temperature.
latent heat of fusion and vaporisation
The specific latent heat of fusion refers to when a substance changes from a solid to a liquid (or vice versa).
The specific latent heat of vaporisation refers to when a substance changes from a liquid to a gas (or vice versa).
particles in gas
Gas particles in a container move around randomly, bouncing off other particles and the walls of the container.
how volume affects pressure in a gas
Decreasing the volume of the container, whilst keeping the number of gas particles the same, will increase the concentration.
In this smaller volume, collisions between particles of gas and the walls of the container will be more frequent.
A greater number of collisions per unit area of wall means the pressure increases.
how temperature and concentration affect a containers flexibble walls
Some containers, such as balloons, are flexible.
An increase in force on the walls of the container would just cause the container to expand.
Therefore, changing temperature or concentration will change the volume of the container, rather than the pressure of the gases inside.