Shapes of simple molecules and ions (3.1.3.5)
1/10
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
11 Terms
What do the dashes and wedges mean in molecule shapes?
Dashes - bond going into paper
Wedges - bond coming out of paper
What is the shape and bond angles of a molecule with 2 bonding pairs?
Shape - linear
Bond angles - 180°

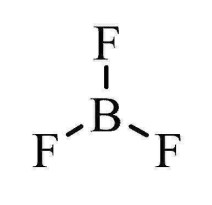
What is the shape and bond angles of a molecule with 3 bonding pairs?
Shape - trigonal planar
Bond angles - 120°
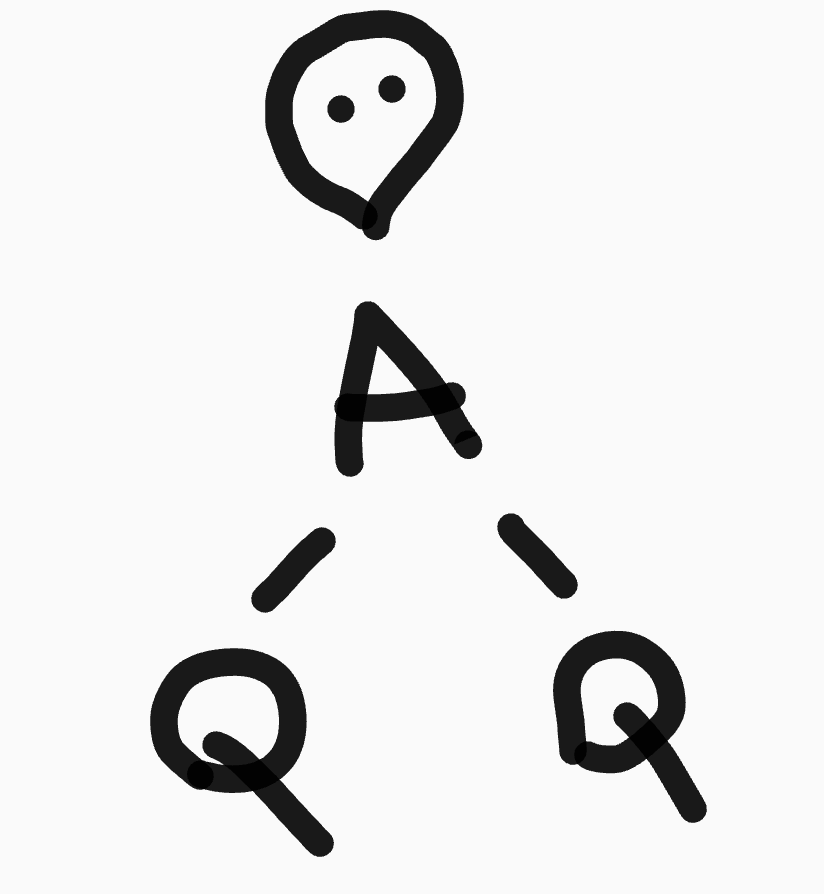
What is the shape and bond angles of a molecule with 2 bonding pairs and 1 lone pair?
Shape - bent (v-shape)
Bond angles - 118°
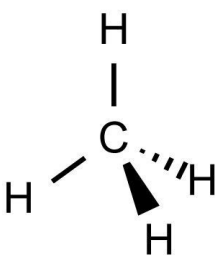
What is the shape and bond angles of a molecule with 4 bonding pairs?
Shape - tetrahedral
Bond angles - 109.5°
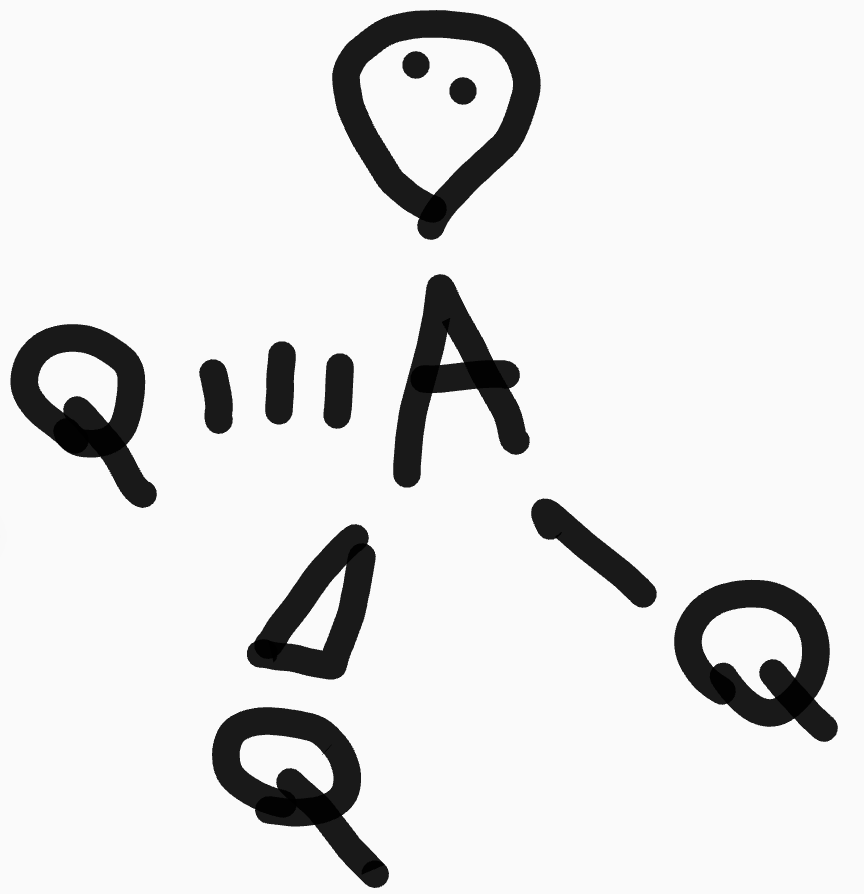
What is the shape and bond angles of a molecule with 3 bonding pairs and 1 lone pair?
Shape - trigonal pyramidal
Bond angles - 107°
What is the shape and bond angles of a molecule with 2 bonding pairs and 2 lone pairs?
Shape - bent (v-shape)
Bond angles - 104.5°
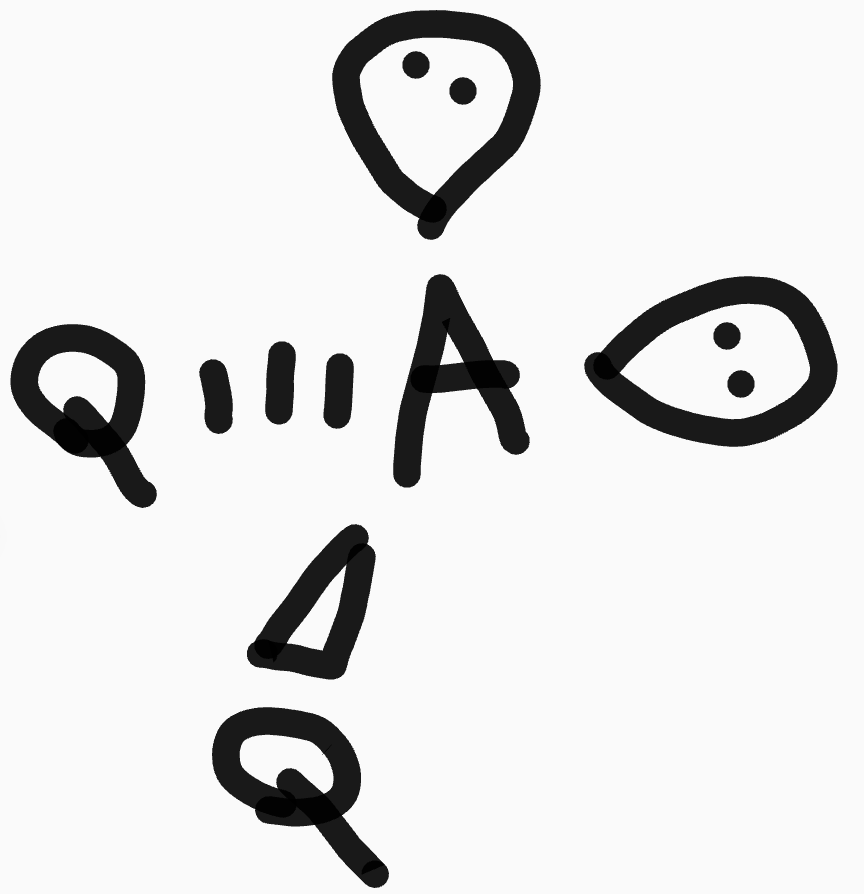
What is the shape and bond angles of a molecule with 5 bonding pairs?
Shape - trigonal bipyramidal
Bond angles - 90° and 120°
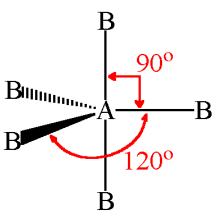
What is the shape and bond angles of a molecule with 6 bonding pairs?
Shape - octahedral
Bond angles - 90°
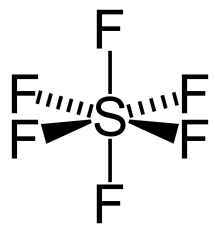
What is the shape and bond angles of a molecule with 4 bonding pairs and 2 lone pairs?
Shape - square planar
Bond angles - 90°
Why do molecules take these shapes?
Molecules take these shapes to minimise electron-electron repulsion
Lone pairs repel more than bonding pairs so it increases the bond angle when lone pairs are present
Bonding pairs repel equally