Biochem Exam 2
1/128
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
129 Terms
- RT lnKeq
-ΔG°’ = ?
ADP
Glutamate
Pi
NH3+
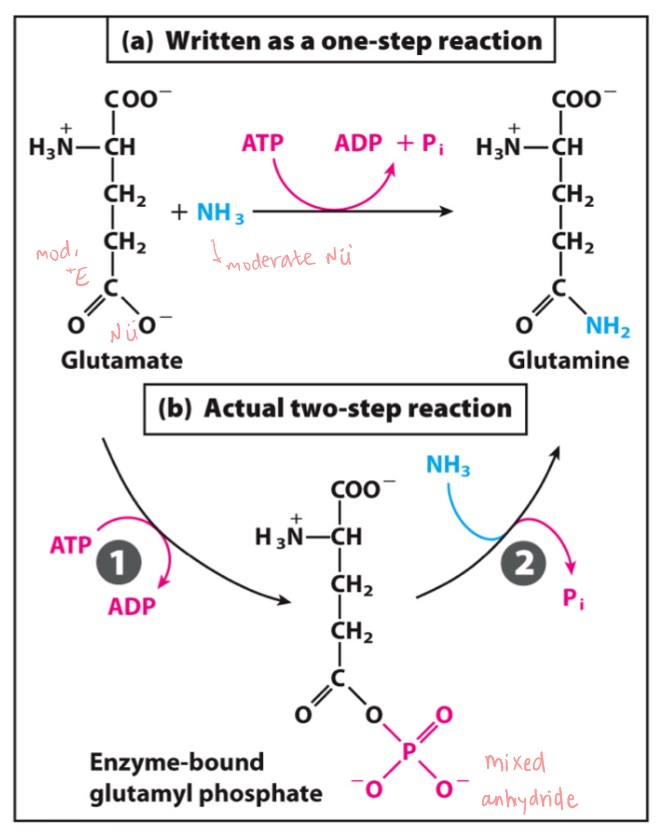
ATP Hydrolysis catalyzed by ATP-dependent glutamine synthetase.
LG in rxn 1?
Nu in rxn 1?
LG in rxn 2?
Nu in rxn 2?
ATP donates its phosphate group directly to substrate/metabolite
Glucose + ATP → Glucose-6-phosphate + ADP (first step of glycolysis).Transfer phosphate to make phosphorylated intermediate
(ATP phosphorylates the intermediate, not the final substrate. Later, that intermediate passes its phosphate to something else:
ATP + Creatine → Creatine-P + ADPLater: Creatine-P + ADP → ATP + Creatine)
Hydrolysis of ATP by protein/enzyme (via conformational changes from bound ligands)
(ATP binds to protein → hydrolysis (ATP → ADP + Pi) makes the protein change shape.That shape change does mechanical or transport work.
Examples:
Myosin (muscle contraction).
Na⁺/K⁺ ATPase pump (ions moved across membrane)
“indirect” ATP hydrolysis
(2 chemical steps:
Step 1: ATP phosphorylates a protein (ATP → ADP, protein gets a phosphate group).Step 2: Later, water removes that phosphate (protein-P → protein + Pi).
The protein’s on/off state depends on whether it’s phosphorylated.
Example: Regulatory enzymes in signaling pathways, like glycogen phosphorylase kinase.)
4 ways of harnessing potential energy from ATP
thermodynamically ; kinetically
(Hydrolysis, ATP → ADP + Pi, is energetically favorable (negative ΔG, releases energy)
(Thermodynamically stable = its breakdown is favorable)
(Kinetically stable: The reaction doesn’t happen quickly on its own because there’s a high activation energy barrier)
ATP is metastable, meaning it is _ unstable, and _ stable.
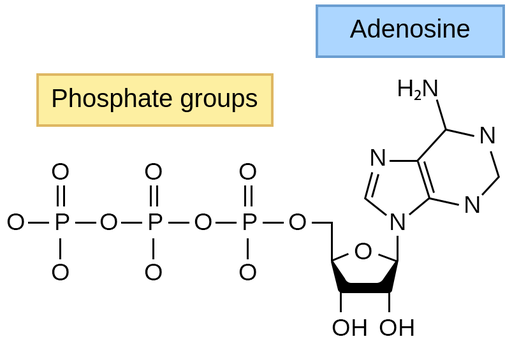
Adenosine Triphosphate
ATP stands for
remove products
(if a product is continuously removed the equilibrium shifts forwardExample: In glycolysis, glucose-6-phosphate is quickly converted into fructose-6-phosphate, keeping the earlier reaction going forward)
Couple w ATP
(If a reaction is energetically unfavorable + ΔG, the cell pairs it with ATP hydrolysis ΔG << 0The combined reaction has an overall negative ΔG → making it favorable
Example: phosphorylation step
Glucose + ATP → Glucose-6-phosphate + ADP )
Two ways to make an unfavorable reaction proceed? (shift eq?)
NO
(a large ΔG°′ doesn’t automatically mean non-spontaneous in the cell, because the cell manipulates concentrations to drive reactions forward.
ΔG°′ (standard free energy change)
Measured at 1 M for all reactants/products, pH 7, 25°C.
Cells rarely have 1 M concentrations.)
Does a large -ΔG°′ mean the reaction is spontaneous in the cell?
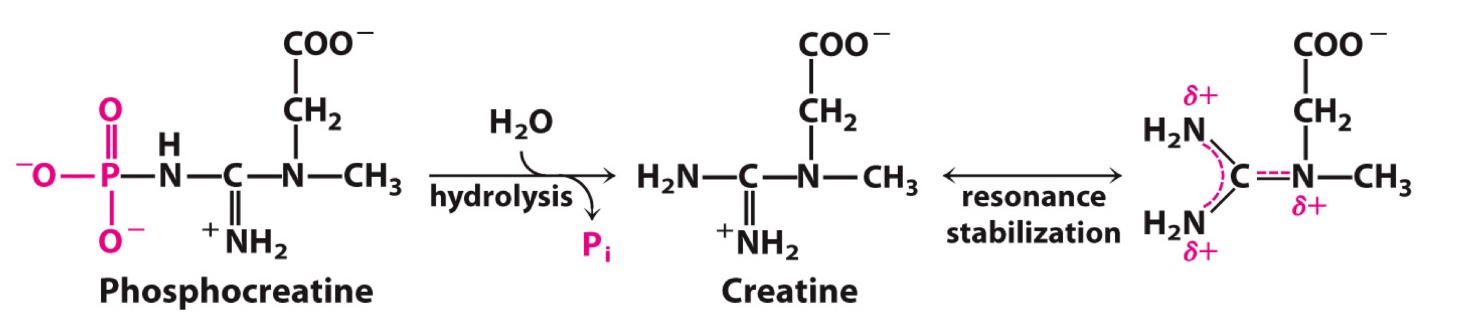
−43
Standard free energy change of creatine phosphate reaction?
Creatine-P + ADP → Creatine + ATP
ΔG°′ = __ kJ/mol
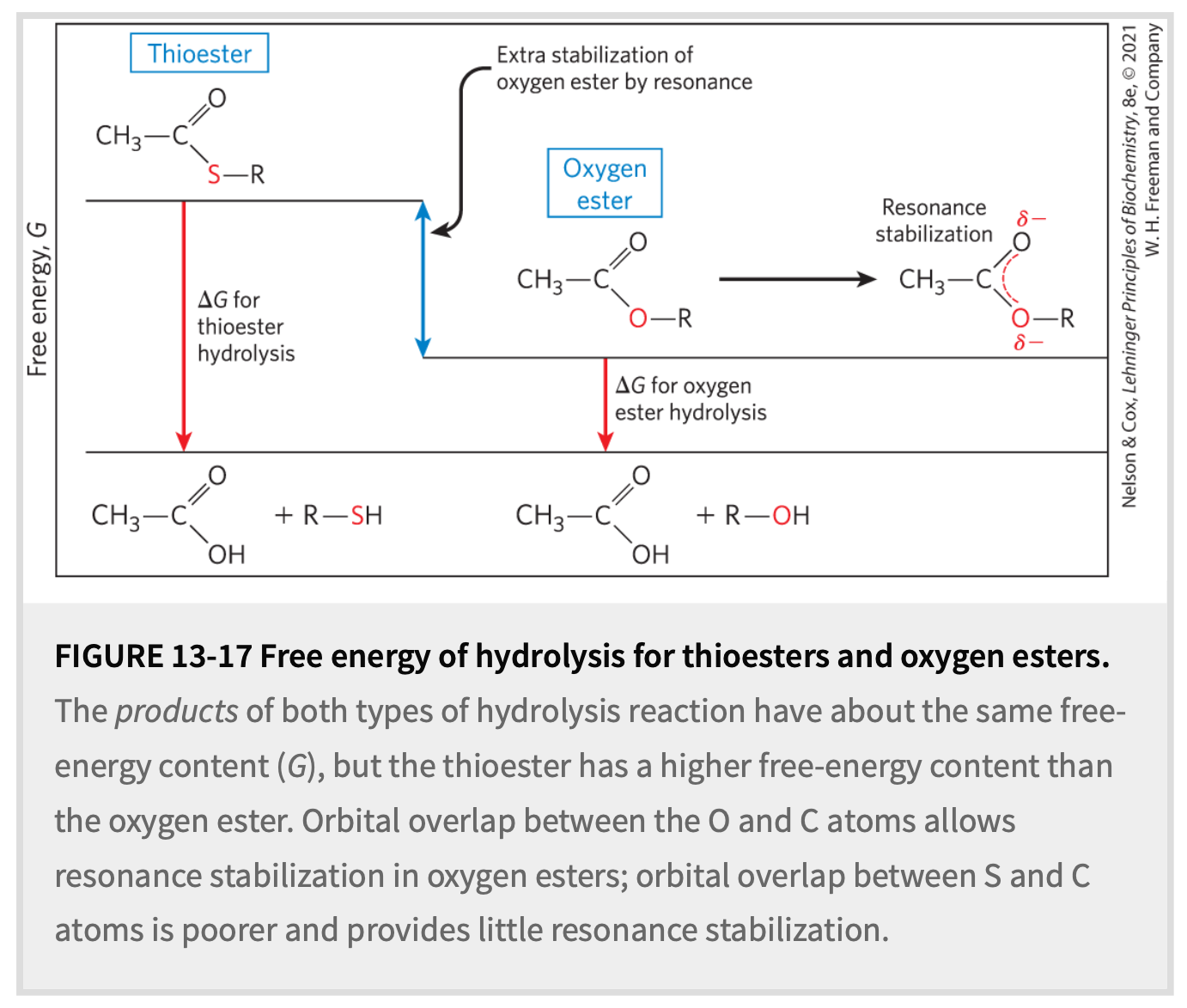
Better leaving group since S- more stable than O-
S less EN, so carbonyl is a better E+
What makes thioesters’’ hydrolysis more favorable than esters?
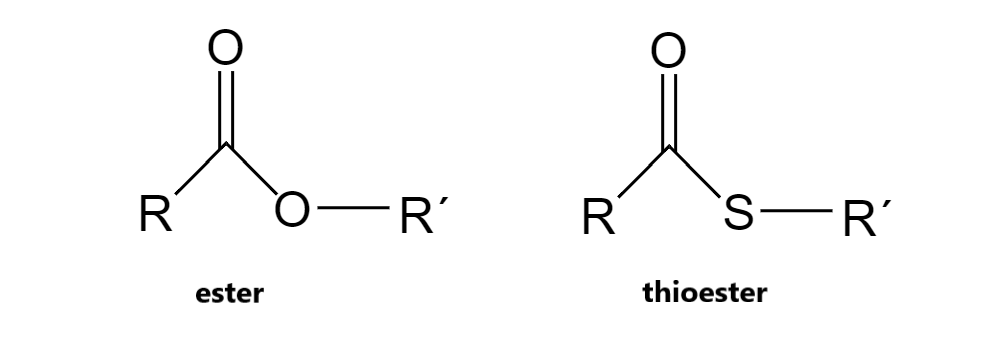
-31
Standard free energy change of thioester hydrolysis?
ΔG°′ = __ kJ/mol
(ATP is ~ -30.5 kJ/mol)
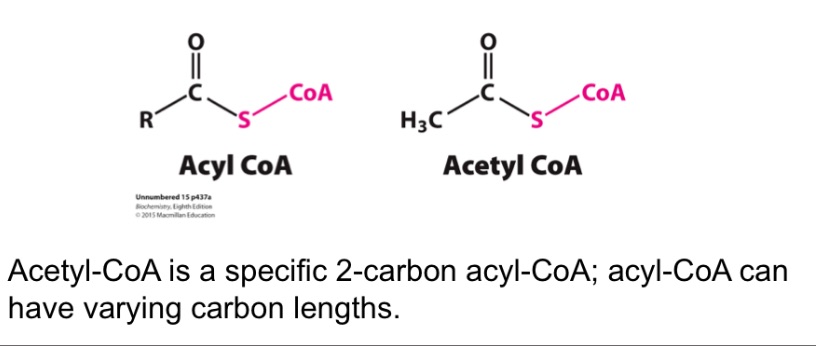
Acetyl-CoA vs Acyl-CoA
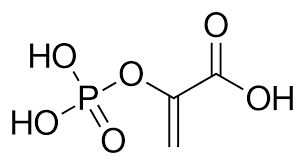
Phosphoenolpyruvate
Name the molecule?
nicotinamide adenine dinucleotide
NAD stands for?
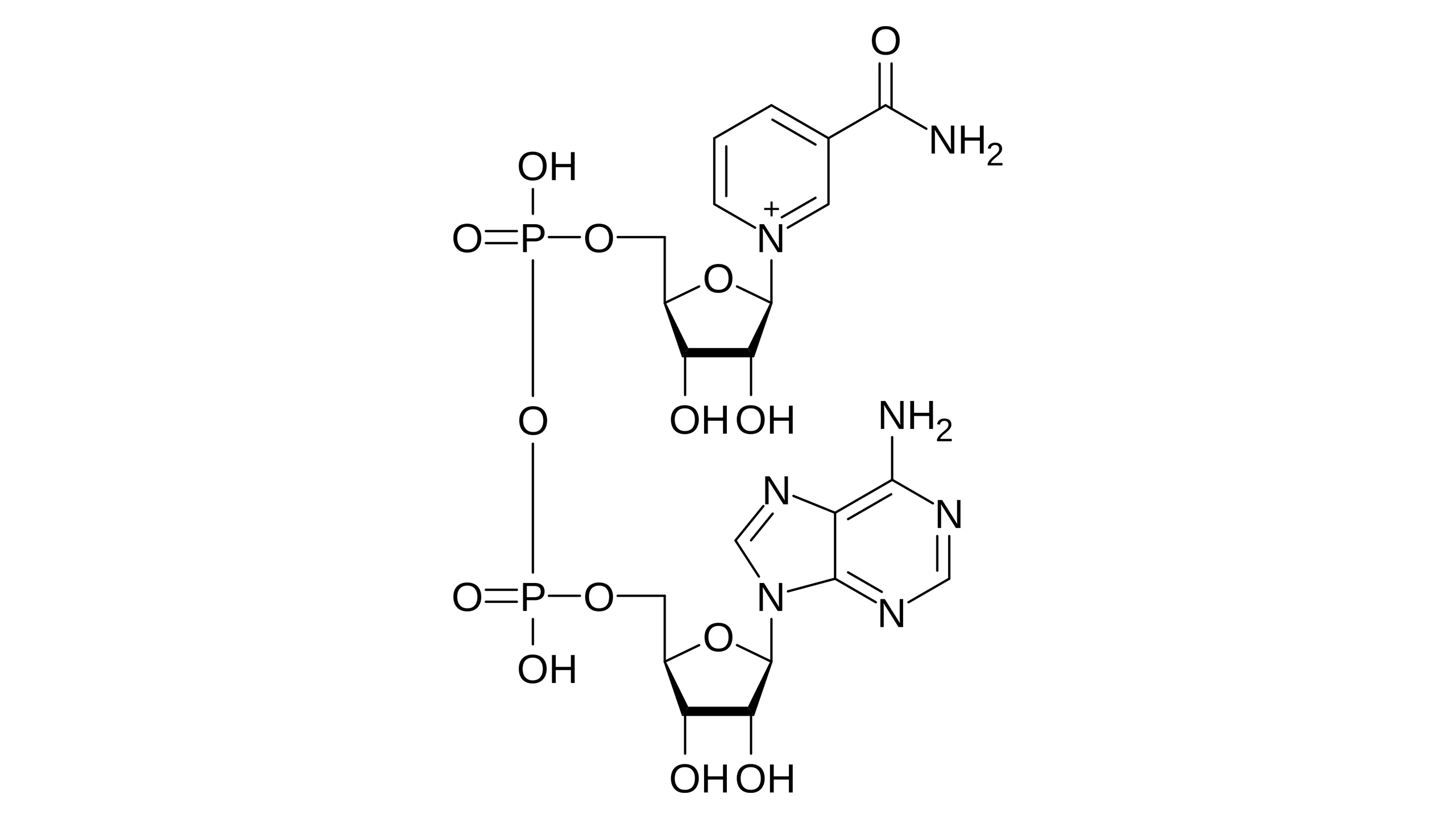
NAD
__ is an obligatory two electron carrier (hydride transfer)
flavin adenine dinucleotide
FAD stands for?
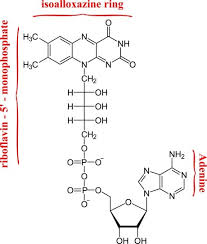
FAD
__ is a two electron carrier, but can take one electron at a time
NADH ; FAD
__ is a mobile electron carrier, __ is often a prosthetic group (not mobile)
riboflavin
__ is B2, obtained in diet
ATP: phosphate donor, product is FMN
ATP is adenylate donor, product is FAD
FAD Synthesis
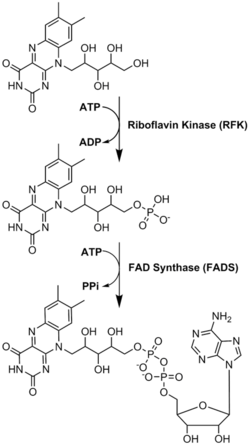
PPi is immediately hydrolyzed by pyrophosphatase
(hydrolysis is strongly exergonic (ΔG°′ ≈ −33 kJ/mol) —> overall ΔG becomes negative)
What happens to PPi product of FAD synthetase?
FAD synthetase reaction:
ATP donates an adenylyl group to FMN → forms FAD
PPi (pyrophosphate) is released as a byproduct
Cn(H2O)n
Carbohydrate formula
glycosidic
___ bond is a covalent bond between the anomeric carbon of a sugar and the hydroxyl group of another molecule (often another sugar).
penultimate
D
L
__ carbon determines if L or D
if OH on right, it’s _
if OH on left, it’s _
RLR(R)
D-Glucose fischer projection:
LLR(R)
D-Mannose fischer projection:
RLL(R)
D-Galactose fischer projection:
epimers
any 2 sugars that differ in configuration around a single carbon
fructose
__ is the ketose isomer of glucose
ribulose
__ is the ketose isomer of ribose
alpha ; beta
in the __ configuration, the anomeric C points DOWN with respect to flag ;
in the __ configuration, the anomeric C points UP
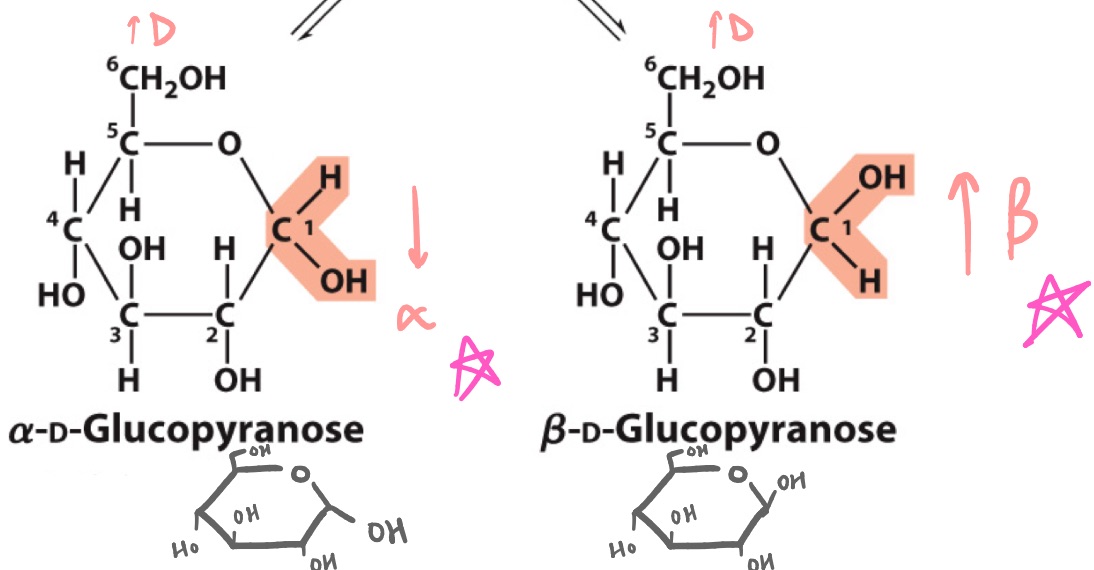
draw alpha- and beta- D-glucopyranose
beta-pyranose
_ is the most stable configuration of glucose
No
Is sucrose a reducing sugar?
yes
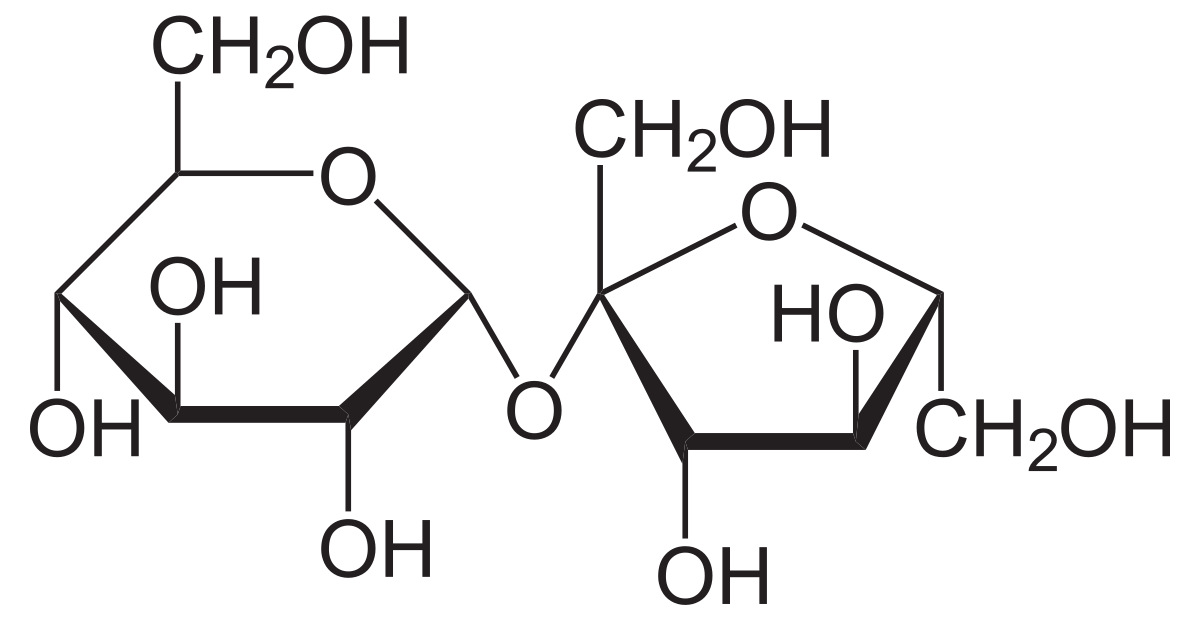
Is lactose a reducing sugar?
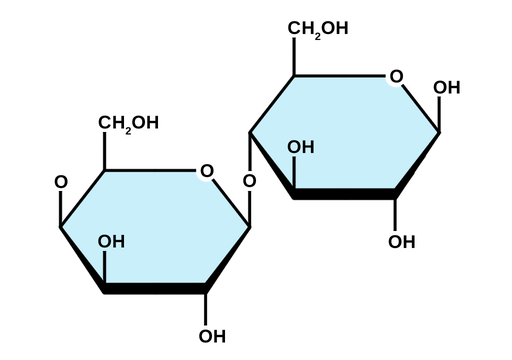
yes
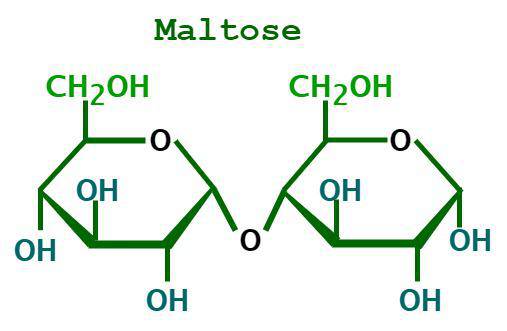
is maltose a reducing sugar?
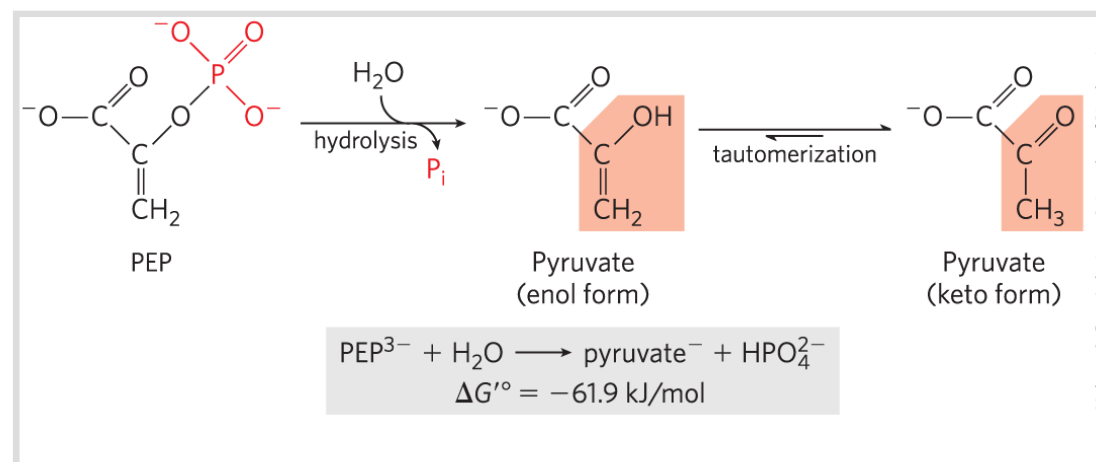
Phosphoenolpyruvate ; PEP
Because ___ (___) has only one form (enol) and the product (pyruvate) has two possible forms, the reaction occurs with a gain in entropy, and the product is therefore stabilized relative to the reactant
Glucose + ATP → Glucose-6-phosphate + ADP
by Hexokinase
Step 1 of Glycolysis
Glucose 6-phosphate isomerized to Fructose 6-phosphate
by phosphoglucose isomerase
Step 2 of Glycolysis
Fructose-6-phosphate + ATP → Fructose-1,6-bisphosphate + ADP
by Phosphofructase-1 (PFK1)
Step 3 of Glycolysis
Fructose-1,6-bisphosphate is split into 2 isomers:
Glyceraldehyde 3-phosphate (G3P)
Dihydroxyacetone phosphate (DHAP)
by aldolase
Step 4 of Glycolysis
Dihydroxyacetone phosphate (DHAP) isomerized to Glyceraldehyde 3-phosphate (G3P)
by Triose Phosphate Isomerase (TPI)
Step 5 of Glycolysis
G3P/NAD+ oxidized/reduced to 1,3-bisphosphoglycerate + NADH
by GAPDH (glyceraldehyde 3-phosphate dehydrogenase)
Step 6 of Glycolysis
1,3-bisphosphoglycerate + ADP → 3-phosphoglycerate + ATP
by phosphoglycerate kinase
Step 7 of Glycolysis
3-phosphoglycerate → 2-phosphoglycerate
by phosphoglycerate mutase
Step 8 of Glycolysis
2-phosphoglycerate → phosphoenolpyruvate (PEP) + H2O
by enolase
Step 9 of Glycolysis
PEP → pyruvate + ATP
by pyruvate kinase
Step 10 of Glycolysis
Starch: alpha1-4 linkages of glucose
Cellulose: Beta1-4 linkages of glucose
Starch vs Cellulose?
In aerobic conditions
Pyruvate → Acetyl-CoA → Citric Acid Cycle → CO₂ + H₂O
In anaerobic conditions
Lactic acid fermentation (muscle cells):
Pyruvate + NADH −> Lactate + NAD+Alcohol (ethanol) fermentation (yeast):
Pyruvate −> Acetaldehyde −> Ethanol + NAD+
(Anabolic uses: Pyruvate is also a precursor for biosynthesis (e.g., amino acids, fatty acids), not just energy metabolism.)
Pyruvate’s Fates
Cori cycle
In muscles, lactate can later be transported to the liver and converted back to glucose via gluconeogenesis (which can then be shipped back to the muscles to fuel more ATP production).
Muscle: glucose → lactate + 2 ATP
Liver: lactate → glucose (consumes ATP)
This is called the _
2 ; 2
2 ; 2 ; 2 ; 2
Glycolysis produces __ NADH per glucose (from _ molecules of glyceraldehyde-3-phosphate).
The reduction of _ pyruvates → _ lactate consumes _ NADH, regenerating _ NAD⁺.
glycolysis
Fermentation itself does not generate ATP; it just allows __ to continue.
Muscle cells continue making ATP anaerobically, producing more lactate than the liver can process. Lactate cannot immediately leave the muscle fast enough, especially if blood flow and oxygen supply are limited.
This leads to lactic acid buildup, which lowers pH inside the muscle (acidification).
Acidification affects enzyme activity and limits muscle performance, causing fatigue.
Why does lactate accumulate in muscle during intense exercise?
Pyruvate → acetaldehyde + NADH (from GAPDH) + CO₂ → ethanol + NAD+
Alcoholic Fermentation Pathway (start from pyruvate)
Glucose → 2 pyruvate → 2 lactate
Pyruvate gets reduced to lactate by TPP, NADH gets oxidized to NAD+
Lactic Acid Fermentation (start from glucose)
TPP; lipoamide; CoA; FAD; NAD⁺
(TPP decarboxylates pyruvate, lipoamide carries the acetyl group, CoA accepts it forming acetyl-CoA, FAD reoxidizes lipoamide, and NAD⁺ is reduced to NADH.)
The coenzymes of PDH act in a specific order:
1.
decarboxylates pyruvate → forms hydroxyethyl-TPP
swings acetyl group and transfers electrons, accepts hydroxyethyl → forms acetyl-lipoamide
accepts acetyl group → forms acetyl-CoA
reoxidizes lipoamide
accepts electrons from FADH₂ → forms NADH
IN PDH, write functions
TPP (thiamine pyrophosphate) –
Lipoate –
Coenzyme A (CoA) –
FAD –
NAD⁺ –
thiamine pyrophosphate
TPP stands for
Enzyme | Function | Prosthetic/Coenzyme Group |
|---|---|---|
E1: Pyruvate dehydrogenase | Decarboxylates pyruvate | TPP (noncovalently bound) |
E2: Dihydrolipoyl transacetylase | Transfers acetyl group to CoA | Lipoyllysyl arm (amide-linked lipoate) |
E3: Dihydrolipoyl dehydrogenase | Regenerates oxidized lipoamide → FAD/NAD⁺ complete electron transfer | FAD tightly bound |
3 enzymes of PDH
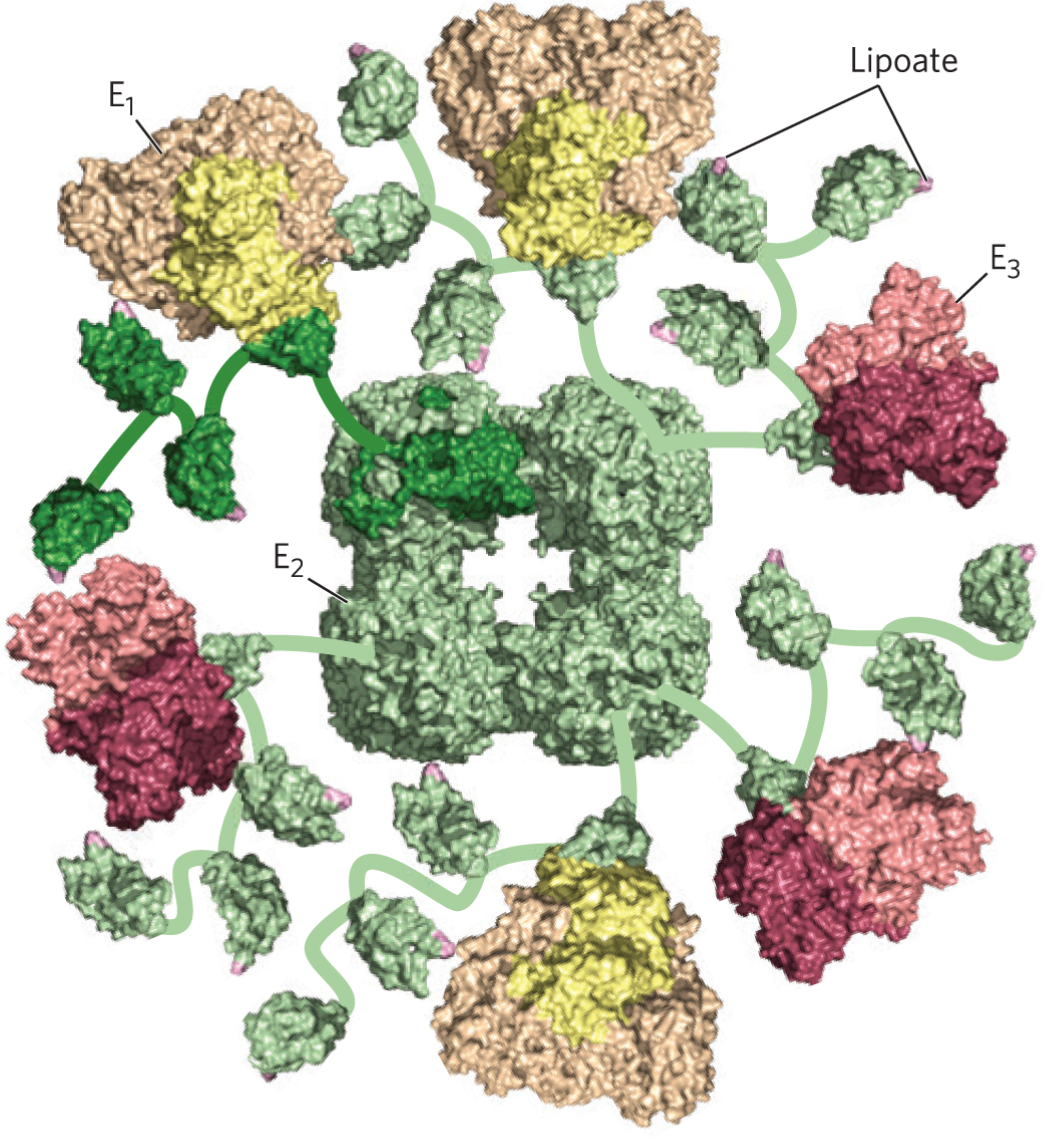
Step | Enzyme | Reaction | Key Details |
|---|---|---|---|
1 | E1: Pyruvate dehydrogenase | Pyruvate + TPP → hydroxyethyl-TPP + CO₂ | Decarboxylation; slowest step → rate-limiting |
2 | E1 → E2 transfer | Hydroxyethyl-TPP → acetyl-lipoamide | Oxidation of hydroxyethyl to acetyl; electrons reduce disulfide of lipoyl group |
3 | E2: Dihydrolipoyl transacetylase | Acetyl-lipoamide + CoA → acetyl-CoA + reduced lipoamide | Transesterification; forms high-energy thioester (acetyl-CoA) |
4 | E3: Dihydrolipoyl dehydrogenase | Reduced lipoamide → FADH₂ | Restores oxidized disulfide form of lipoyl group |
5 | E3: Dihydrolipoyl dehydrogenase | FADH₂ + NAD⁺ → FAD + NADH | Transfers electrons to NAD⁺; completes catalytic cycle |
Pyruvate enters E1 → loses CO₂ → attaches to TPP
TPP passes acetyl to swinging lipoate arm (E2)
Lipoate transfers acetyl to CoA → forms acetyl-CoA
Lipoate is reoxidized by FAD (E3)
FAD passes electrons to NAD⁺ → NADH
Cycle ready to start again
5 Steps of PDH
lactate; lactate dehydrogenase; NAD⁺
In lactate fermentation, pyruvate is reduced to __________ by __________, which regenerates __________ for glycolysis.
acetaldehyde; pyruvate decarboxylase; ethanol; alcohol dehydrogenase
In ethanol fermentation, pyruvate is first converted to __________ by __________, and then to __________ by __________ using NADH.
During intense exercise, glycolysis breaks down glucose into pyruvate.
[The muscle breaks down glycogen (glycogenolysis) to get glucose-6-phosphate (G6P), G6P enters glycolysis, generating ATP quickly (without needing oxygen]
Because oxygen is low, pyruvate is reduced to lactate by lactate dehydrogenase (via muscle isoform: LDH-M), regenerating NAD⁺ so glycolysis can keep going.
This produces ATP for the muscle, but also lactate, which accumulates and lowers pH (causing fatigue).
The lactate is then released into the bloodstream and transported to the liver.
The liver takes up lactate and converts it back into pyruvate (via liver isoform: LDH-H).
Then, the liver does gluconeogenesis, converting pyruvate → glucose.
This glucose is released into the blood and delivered back to the muscles (stored as glycogen, or used again for energy).
You using the Cori Cycle!
1, 3, 10
3 Irreversible steps of glycolysis?
Glucose-6-phosphatase enzyme
G6P —> Glucose
by what enzyme ?
Fructose-1,6-bisphosphatase
F1,6 Bisphosphate —> F6P
by what enzyme ?
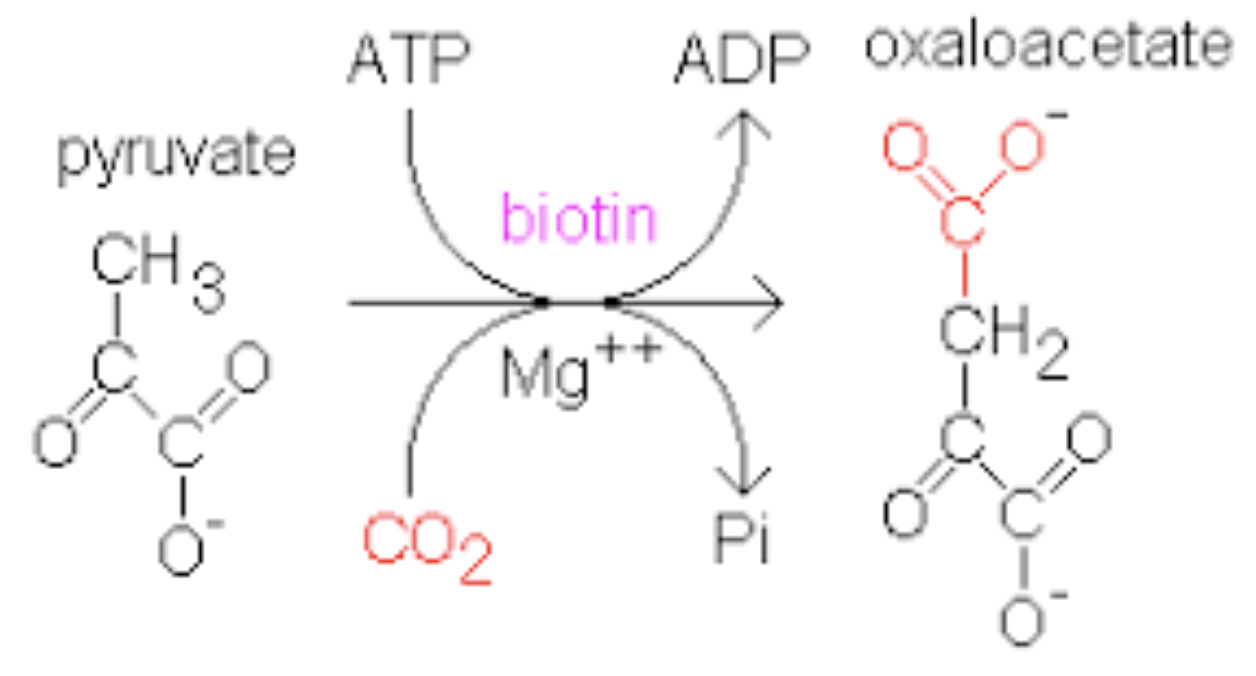
pyruvate carboxylate + BIOTIN COFACTOR (CO2 carrier)
Pyruvate —> oxaloacetate
by what enzyme ?
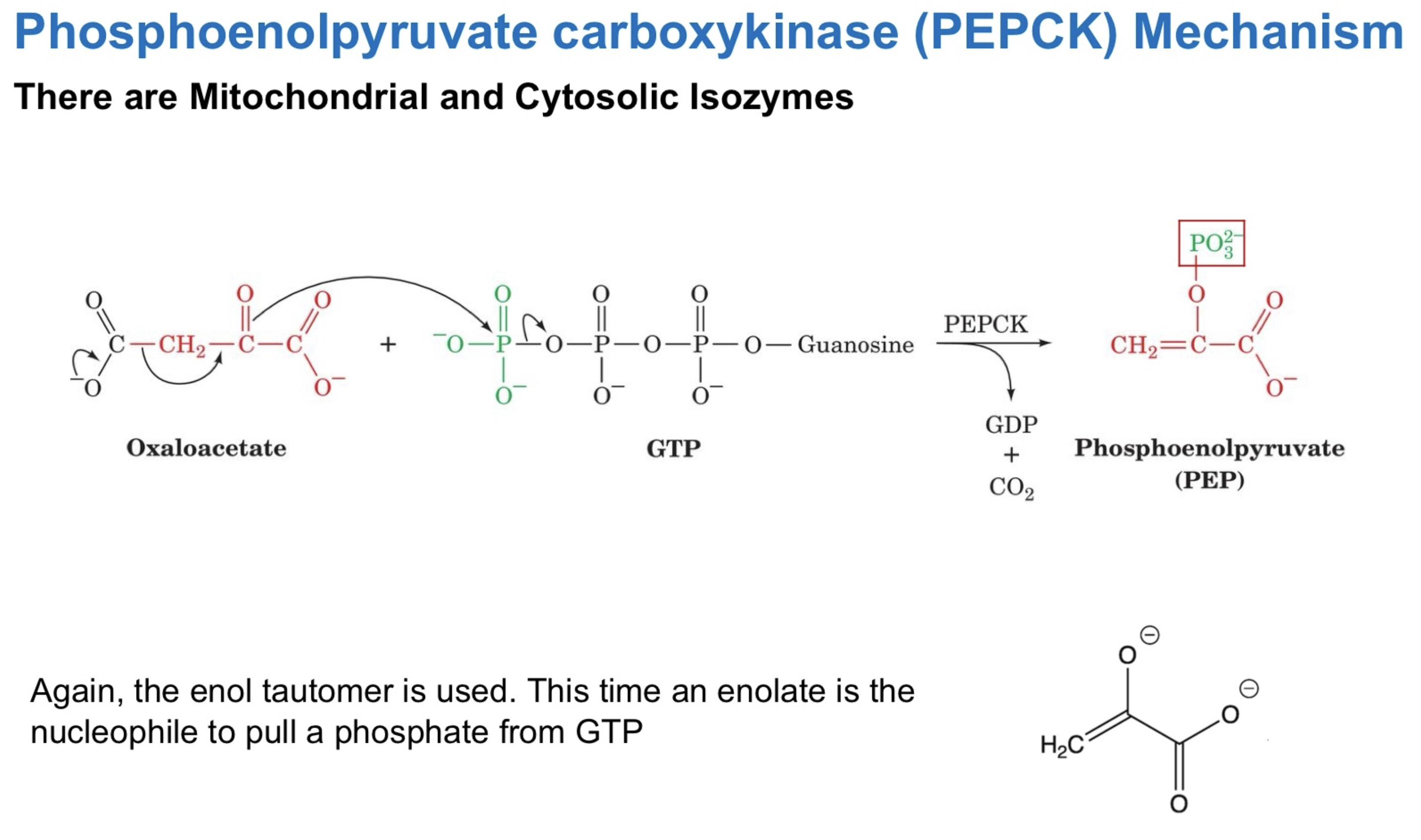
PEPCK (Phosphoenolpyruvate carboxykinase) USES GTP
oxaloacetate —> PEP
by what enzyme ?
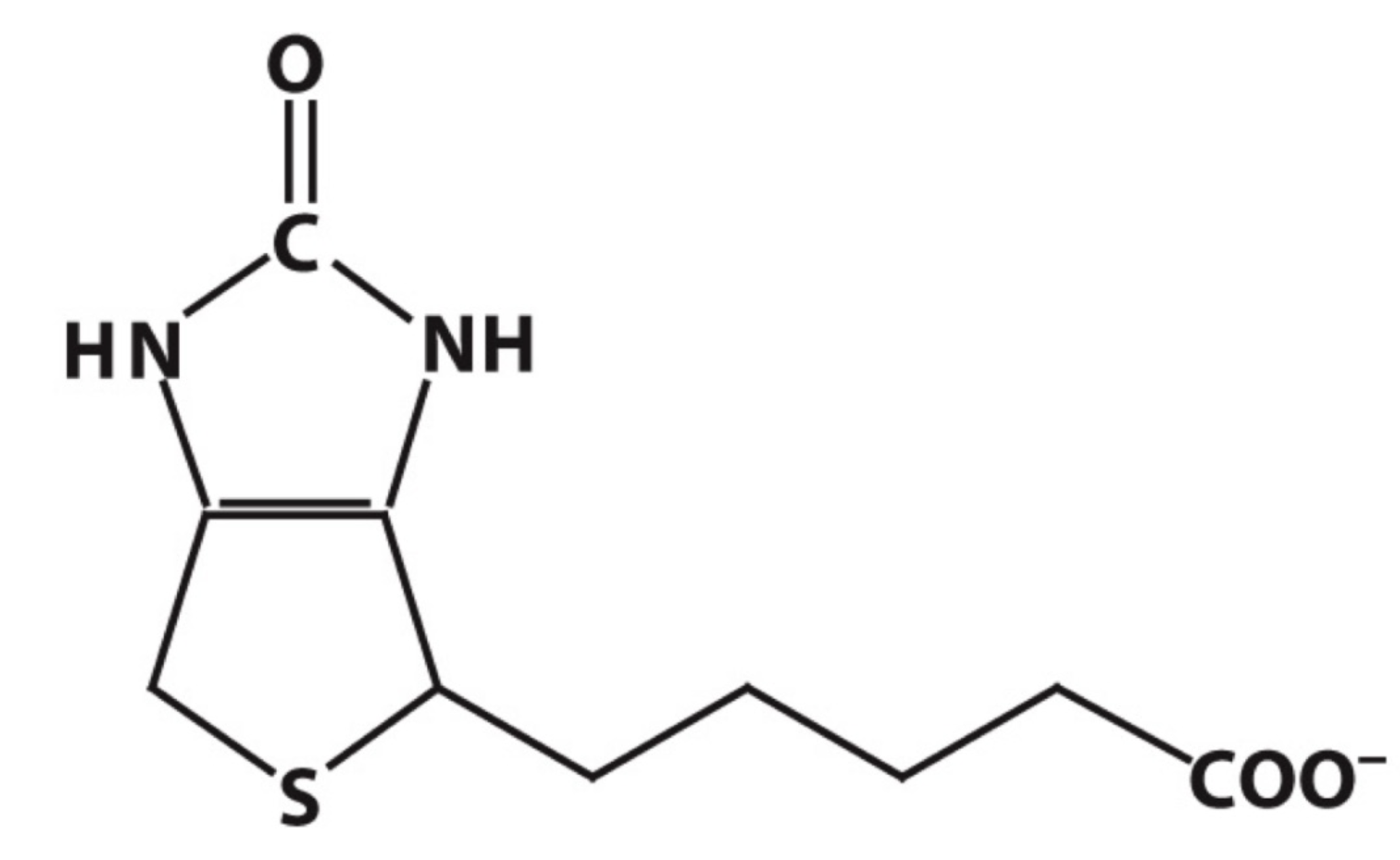
biotin
WHAT THAT?
Amino acids ; fatty acids (plants/bacteria can tho)
Mammals can generate glucose from _ NOT from _
acetyl-CoA
Why Mammals Can’t Convert Fatty Acids → Glucose:
Fatty acid breakdown (β-oxidation) produces acetyl-CoA.
To make glucose, you’d need to convert acetyl-CoA → pyruvate → oxaloacetate → glucose.
But the pyruvate dehydrogenase (PDH) reaction is irreversible:
𝑃𝑦𝑟u𝑣𝑎𝑡𝑒 + 𝐶𝑜𝐴 + 𝑁𝐴𝐷+ → 𝐴𝑐𝑒𝑡𝑦𝑙 − 𝐶𝑜𝐴 + 𝐶𝑂2 + 𝑁𝐴𝐷𝐻
Once carbon becomes __, it cannot go back to pyruvate or oxaloacetate in mammals, and in TCA cycle, the Cs from it get lost as CO2
But plants/yeast/bacteria can convert acetyl-CoA → succinate → oxaloacetate → glucose using the glyoxylate cycle.
2 pyruvate + 4 ATP + 2 GTP + 2 NADH + 2H+ + 4 H2O —>
Glucose + 4 ADP + 2GDP + 6 Pi + 2 NAD+
Gluconeogenesis overall equation:
starvation
vigorous exercise
Two ways glycogen stores can get depleted?
glucose because G6P too charged to cross membranes.
(Liver uses G6Pase in ER to irreversibly remove phosphate from glucose, now can bind to GLUT2 transporters to exit liver cell to bloodstream)
Can sugar leave the liver as G6P or glucose? Why?
leave the cell
Without G6Pase, the liver could make G6P, but G6P can’t ___ because:
It’s negatively charged
There’s no transporter for phosphorylated sugars across plasma membranes
ER lumen
By having G6Pase sequestered in the __ ____, this prevents the enzyme from accidentally burning through all cytosolic G6P and regulate how much glucose leaves the liver.
Pyruvate carboxylase uses the vitamin biotin as a cofactor. The formation of oxaloacetate by pyruvate carboxylase occurs in three stages. The biotin carboxylase domain catalyzes the formation carboxyphosphate. The carboxylase then transfers the CO2 to the biotin carboxyl carrier protein (BCCP). The BCCP carries the activated CO2 to the transcarboxylation (CT) domain, where the CO2 is transferred to pyruvate to form oxaloacetate.
Fructose-1,6-bisphosphatase; Phosphofructokinase-1 (PFK-1)
The enzyme that converts fructose-1,6-bisphosphate → fructose-6-phosphate in gluconeogenesis is called ____________, and this step bypasses the enzyme ____________ from glycolysis.
B
(To make 1 molecule of glucose, gluconeogenesis consumes 4 ATP + 2 GTP + 2 NADH)
5. Which of the following statements about the energetics of gluconeogenesis is TRUE?
A) It releases energy overall.
B) It requires more energy than glycolysis generates.
C) It is energy neutral.
D) It only occurs when oxygen is low.
reciprocally
Both glycolysis and gluconeogenesis are thermodynamically favorable because they are ___ regulated (only one pathway is active at a time)
glucose
Your body can’t always get sugar (glucose) from food, so it makes its own!
It does this by taking other molecules (like lactate, pyruvate, or parts of the citric acid cycle) and turning them backward into __.
But some steps of glycolysis (the process that breaks down glucose) are one-way only — they release too much energy to reverse.
So your body uses special “bypass” enzymes to go around them, these are the three irreversible steps.
Glucose-6-phosphatase
Hexokinase bypass enzyme?
Fructose-1,6-bisphosphatase (FBPase-1)
Phosphofructokinase-1 (PFK-1) bypass enzyme?
Pyruvate carboxylase+biotin & PEP carboxykinase+GTP
Pyruvate kinase bypass enzymes?
Pyruvate carboxylase
PEP carboxykinase
Fructose-1,6-bisphosphatase
Glucose-6-phosphatase
mnemonic for the bypass enzymes:
“Path Produces Fructose (then) Glucose”
C
(others are either allosterically regulated to a lesser extent (pyruvate carboxylase) or mainly transcriptionally controlled (PEPCK, G6Pase)
Which step is the major regulation point in gluconeogenesis?
A) Pyruvate → Oxaloacetate
B) Oxaloacetate → PEP
C) Fructose-1,6-bisphosphate → Fructose-6-phosphate
D) Glucose-6-phosphate → Glucose
I-III
Muscle Hexokinase numbers
IV
Liver hexokinase number
I-III: High affinity for glucose (Km ≈ 0.2 mM), Inhibited by product G6P
IV: Low affinity for glucose (Km ≈ 10 mM), Not inhibited by G6P, Transcriptionally regulated by energy needs or blood glucose levels.
Compare hexokinase I-III with IV
gluconeogenesis
High ATP, high citrate → PFK-1 inhibited, FBPase-1 active → favors ___.
glycolysis
high AMP → PFK-1 active, FBPase-1 inhibited → favors ___.
Glucokinase = Hexokinase IV!
Hexokinase, but not ___ (liver one), is inhibited by G6P
ATP
In PFK-1 allosteric regulation, _ is both a substrate and a negative allosteric effector
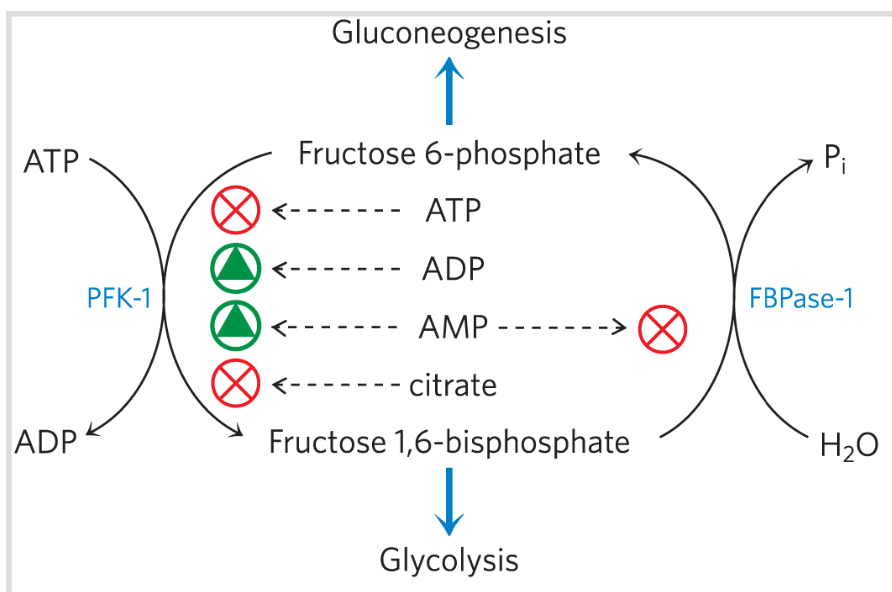
AMP & ADP
Fructose 2,6-bisphosphate (F2,6BP)
Activators of Reaction catalyzed by PFK-1?
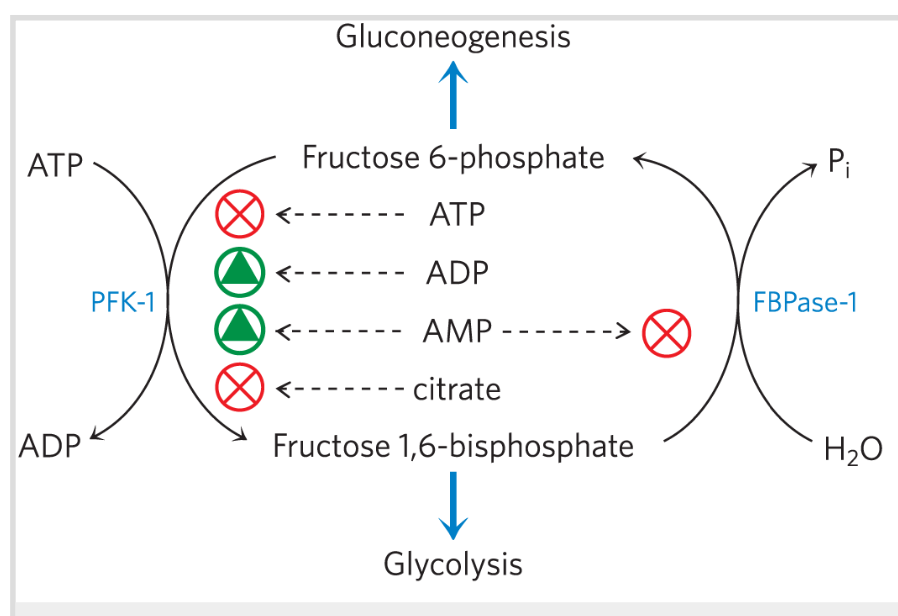
ATP, Citrate
Inhibitors of Reaction catalyzed by PFK-1?
Fructose 2,6-bisphosphate (F2,6BP)
What is…
Produced by PFK-2.
Very strong allosteric activator of PFK-1.
is a signal of high blood glucose.
Shifts enzyme to high-activity state (R), even if ATP is high.
F2,6bPase
PFK2 is the same enzyme as __
Glucagon
__ → lowers F2,6BP → inhibits glycolysis, activates gluconeogenesis → raises blood glucose
pyruvate
Alanine can easily be converted to __
pyruvate carboxylase
Acetyl-CoA stimulates glucose synthesis by activating __
glycogen degredation
epinephrine and glucagon stimulate __