unit 5 review
1/52
Earn XP
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
53 Terms
How are ions formed ?
Ions are formed when atoms gain or lose electrons
What change do columns 1,2,13,15,16, and 17 take on what charged?
1 has a +1, 2 has +2, 13 has +3, and 15 has -3, 16 has -2 and 17 has -1 charge
What is the octet rule?
The octet rule is when the atom wants 8 valance electrons.
What elements are okay with more/ less than 8 electrons?
Less than 8 is H, He, LI, Be and B. More than 8 electrons are P, S, CL, Br, L,and Xe
What are the three types of bond?
Ionic, Covalent, and Metallic
What is cation?
It is when the atoms lose electrons, which makes an atom positively charged
What is an anions?
Is when the atom gain electrons, which makes an atoms negatively charged
Which elements require a roman numeral when naming and why?
The transition metals since they are many possible charges.
Which elements are the exceptions from the Roman numerals?
Zinc (Zn) and Sliver (Ag). The sometimes is Cadmium (Cd) and is sometimes +2
What is bond polarity?
Describes how equal or unequal electrons are shared between two atoms
How to determine if a bond is polar ?
If it is share equally then it’s nonpolar, and if it’s share unequally then it’s polar
What are the seven diatomic names?
Iodine, Nitrogen, Chlorine, Hydrogen, Oxygen, Flourine, Bromine
What is Br2 ?
Bromine
What is I2?
Iodine
What is N2?
nitrogen
What is Cl2?
Chlorine
What is H2?
hydrogen
What is O2?
oxygen
What is F2?
fluorine
What is a nonmetal + nonmetal?
covalent
What is a nonmetal + metal?
ionic
What is a metal + metal?
What is metallic state of matter?
A soild
What is Ionic state of matter?
usually soild
What is covalent state of matter?
soild, liquid, or gas
What is metallic melting point?
high
What is Ionic melting point
high
What is covalent melting point?
low
What is metallic solubility?
insoluble
What is ionic solubility?
usually soluble
What is Covalent solubility?
low for most
Is metallic a good conductor for electricity?
excellent conductor
Is ionic a good conductor for electricity?
yes when dissolved in water, yes when melted, and no as a soild
Is covalent a good conductor for electricity?
no
What is momo?
1
What is di?
2
What is tri?
3
What is tetra?
4
What is penta?
5
What is hexa?
6
What is hepta?
7
What is octa?
8
What is SO42- ?
sulfate
What is NH4+1 ?
ammonium
What is OH-1 ?
hydroxide
What is CO3-2 ?
carbonate
What is PO4-3 ?
phosphate
What is NO3-1 ?
nitrate
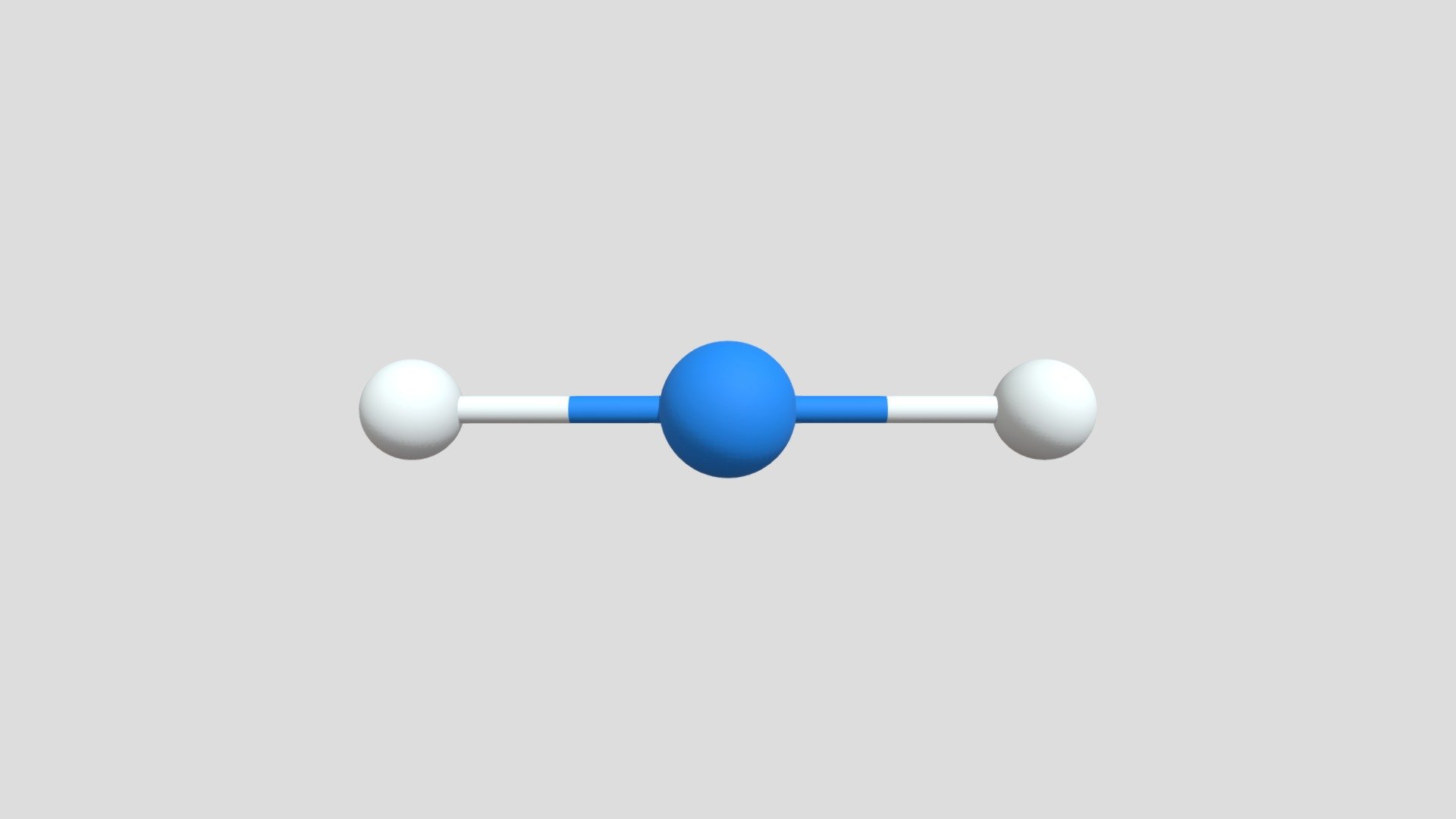
What is the molecular geometry?
linear
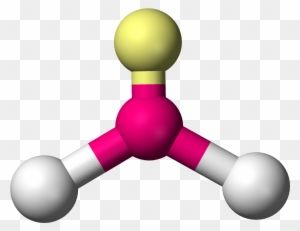
What is the molecular geometry?
trigonal planar
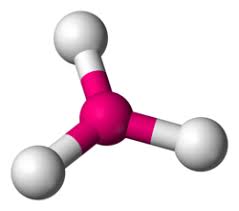
What is the molecular geometry?
trigonal pyramidal
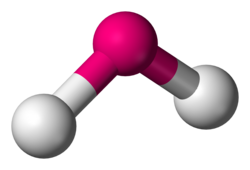
What is the molecular geometry?
bent
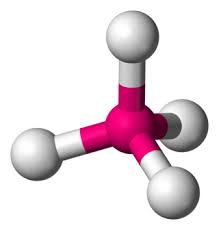
What is the molecular geometry?
tetrahedral